Origin of a Core Bacterial Gene via Co-option and Detoxification of a Phage Lysin
- PMID: 31080080
- PMCID: PMC6594375
- DOI: 10.1016/j.cub.2019.04.032
Origin of a Core Bacterial Gene via Co-option and Detoxification of a Phage Lysin
Abstract
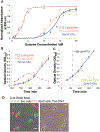
Temperate phages constitute a potentially beneficial genetic reservoir for bacterial innovation despite being selfish entities encoding an infection cycle inherently at odds with bacterial fitness. These phages integrate their genomes into the bacterial host during infection, donating new but deleterious genetic material: the phage genome encodes toxic genes, such as lysins, that kill the bacterium during the phage infection cycle. Remarkably, some bacteria have exploited the destructive properties of phage genes for their own benefit by co-opting them as toxins for functions related to bacterial warfare, virulence, and secretion. However, do toxic phage genes ever become raw material for functional innovation? Here, we report on a toxic phage gene whose product has lost its toxicity and has become a domain of a core cellular factor, SpmX, throughout the bacterial order Caulobacterales. Using a combination of phylogenetics, bioinformatics, structural biology, cell biology, and biochemistry, we have investigated the origin and function of SpmX and determined that its occurrence is the result of the detoxification of a phage peptidoglycan hydrolase gene. We show that the retained, attenuated activity of the phage-derived domain plays an important role in proper cell morphology and developmental regulation in representatives of this large bacterial clade. To our knowledge, this is the first observation of a phage gene domestication event in which a toxic phage gene has been co-opted for core cellular function at the root of a large bacterial clade.
Keywords: Alphaproteobacteria; Asticcacaulis; Caulobacter; GH24 lysozyme; bacterial evolution; prophage domestication.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures

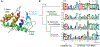


References
-
- Feiner R, Argov T, Rabinovich L, Sigal N, Borovok I, and Herskovits AA (2015). A new perspective on lysogeny: prophages as active regulatory switches of bacteria. Nat. Rev. Microbiol 13, 641–650. - PubMed
-
- Harrison E, and Brockhurst MA (2017). Ecological and Evolutionary Benefits of Temperate Phage: What Does or Doesn’t Kill You Makes You Stronger. BioEssays 39, 1700112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

