Modeling Motor Neuron Resilience in ALS Using Stem Cells
- PMID: 31080111
- PMCID: PMC6565614
- DOI: 10.1016/j.stemcr.2019.04.009
Modeling Motor Neuron Resilience in ALS Using Stem Cells
Abstract
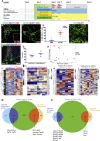
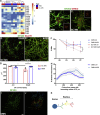
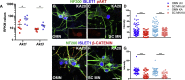
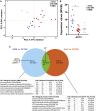
Oculomotor neurons, which regulate eye movement, are resilient to degeneration in the lethal motor neuron disease amyotrophic lateral sclerosis (ALS). It would be highly advantageous if motor neuron resilience could be modeled in vitro. Toward this goal, we generated a high proportion of oculomotor neurons from mouse embryonic stem cells through temporal overexpression of PHOX2A in neuronal progenitors. We demonstrate, using electrophysiology, immunocytochemistry, and RNA sequencing, that in vitro-generated neurons are bona fide oculomotor neurons based on their cellular properties and similarity to their in vivo counterpart in rodent and man. We also show that in vitro-generated oculomotor neurons display a robust activation of survival-promoting Akt signaling and are more resilient to the ALS-like toxicity of kainic acid than spinal motor neurons. Thus, we can generate bona fide oculomotor neurons in vitro that display a resilience similar to that seen in vivo.
Keywords: ALS; LCM sequencing; Onuf’s nucleus; Phox2a; RNA sequencing; amyotrophic lateral sclerosis; neuronal vulnerability and resistance; oculomotor neurons; spinal motor neurons; stem cells.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Boillee S., Yamanaka K., Lobsiger C.S., Copeland N.G., Jenkins N.A., Kassiotis G., Kollias G., Cleveland D.W. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

