Neural Circuitry Underlying Waking Up to Hypercapnia
- PMID: 31080401
- PMCID: PMC6497806
- DOI: 10.3389/fnins.2019.00401
Neural Circuitry Underlying Waking Up to Hypercapnia
Abstract
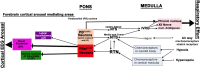
Obstructive sleep apnea is a sleep and breathing disorder, in which, patients suffer from cycles of atonia of airway dilator muscles during sleep, resulting in airway collapse, followed by brief arousals that help re-establish the airway patency. These repetitive arousals which can occur hundreds of times during the course of a night are the cause of the sleep-disruption, which in turn causes cognitive impairment as well as cardiovascular and metabolic morbidities. To prevent this potential outcome, it is important to target preventing the arousal from sleep while preserving or augmenting the increase in respiratory drive that reinitiates breathing, but will require understanding of the neural circuits that regulate the cortical and respiratory responses to apnea. The parabrachial nucleus (PB) is located in rostral pons. It receives chemosensory information from medullary nuclei that sense increase in CO2 (hypercapnia), decrease in O2 (hypoxia) and mechanosensory inputs from airway negative pressure during apneas. The PB area also exerts powerful control over cortical arousal and respiration, and therefore, is an excellent candidate for mediating the EEG arousal and restoration of the airway during sleep apneas. Using various genetic tools, we dissected the neuronal sub-types responsible for relaying the stimulus for cortical arousal to forebrain arousal circuits. The present review will focus on the circuitries that regulate waking-up from sleep in response to hypercapnia.
Keywords: arousal; calcitonin gene related peptide; hypercapnia; obstructive sleep apnea; parabrachial nucleus.
Figures


References
-
- Abbott S. B., Holloway B. B., Viar K. E., Guyenet P. G. (2014). Vesicular glutamate transporter 2 is required for the respiratory and parasympathetic activation produced by optogenetic stimulation of catecholaminergic neurons in the rostral ventrolateral medulla of mice in vivo. Eur. J. Neurosci. 39 98–106. 10.1111/ejn.12421 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

