Enzymatic hydrolysis of starch into sugars is influenced by microgel assembly
- PMID: 31080766
- PMCID: PMC6500924
- DOI: 10.1016/j.btre.2019.e00342
Enzymatic hydrolysis of starch into sugars is influenced by microgel assembly
Erratum in
-
Erratum regarding missing Declaration of Competing Interest statements in previously published articles.Biotechnol Rep (Amst). 2021 Mar 19;29:e00582. doi: 10.1016/j.btre.2020.e00582. eCollection 2021 Mar. Biotechnol Rep (Amst). 2021. PMID: 33786326 Free PMC article.
Abstract
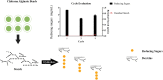
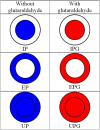
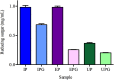
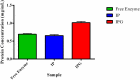
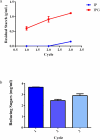
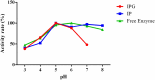
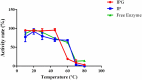
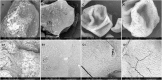
The use of alginate and chitosan polymer in the immobilization of Aspergillus oryzae ATCC 3940 fungal crude enzyme extract (CEE) amylase was presented. The assembly results change in the application of optimal pH and temperature hydrolysis to convert starch to sugar. Bead arrangement in three microgel supports: the internal support phase (IP), the external support phase (EP), and the internal and external support phase (UP). The best results were obtained using IP and EP. Reusing beads evaluated the stability of immobilized enzymes on IP support, remained active and bound during three cycles of reuse. For free and immobilized (IP) activity showed pH ranged from 5.0 to 7.0; optimum thermal enzymatic greater activity at 45 °C. The method of building the microgel influencing sugar reduction, in a single-step way to immobilize crude fungal amylase extracts can be used in industry.
Keywords: Alginate; Beads; Chitosan; Hydrolysis; Reducing sugars; Starch.
Figures

References
-
- Saleem A., Ebrahim M.K. Production of amylase by fungi isolated from legume seeds collected in Almadinah Almunawwarah, Saudi Arabia. J. Taibah Univ. Sci. 2014;8(2):90–97.
LinkOut - more resources
Full Text Sources
