Increased tenofovir monoester concentrations in patients receiving tenofovir disoproxil fumarate with ledipasvir/sofosbuvir
- PMID: 31081036
- PMCID: PMC6640288
- DOI: 10.1093/jac/dkz184
Increased tenofovir monoester concentrations in patients receiving tenofovir disoproxil fumarate with ledipasvir/sofosbuvir
Abstract
Background: Intracellular tenofovir diphosphate concentrations are markedly increased in HIV/HCV coinfected individuals receiving tenofovir disoproxil fumarate (TDF) with sofosbuvir-containing treatment. Sofosbuvir may inhibit the hydrolysis of TDF to tenofovir, resulting in increased concentrations of the disoproxil or monoester forms, which may augment cell loading. We sought to quantify tenofovir disoproxil and monoester concentrations in individuals receiving TDF with and without ledipasvir/sofosbuvir.
Methods: HIV/HCV coinfected participants receiving TDF-based therapy were sampled pre-dose and 1 and 4 h post-dose prior to and 4 weeks after initiating ledipasvir/sofosbuvir. Tenofovir disoproxil was not detectable. Tenofovir monoester in plasma and tenofovir diphosphate in PBMC and dried blood spots (DBS) were quantified using LC-MS/MS. Geometric mean ratios (week 4 versus baseline) and 95% CIs were generated for the pharmacokinetic parameters. P values reflect paired t-tests.
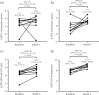
Results: Ten participants had complete data. At baseline, geometric mean (95% CI) tenofovir monoester plasma concentrations at 1 and 4 h post-dose were 97.4 ng/mL (33.0-287.5) and 0.74 ng/mL (0.27-2.06), respectively. With ledipasvir/sofosbuvir, tenofovir monoester concentrations at 4 h post-dose were 5.02-fold higher (95% CI 1.40-18.05; P = 0.019), but did not significantly differ at 1 h post-dose (1.72-fold higher, 95% CI 0.25-11.78; P = 0.54), possibly due to absorption variability. Tenofovir diphosphate in PBMC and DBS were increased 2.80-fold (95% CI 1.71-4.57; P = 0.001) and 7.31-fold (95% CI 4.47-11.95; P < 0.0001), respectively, after 4 weeks of ledipasvir/sofosbuvir.
Conclusions: Tenofovir monoester concentrations were increased in individuals receiving TDF with ledipasvir/sofosbuvir, consistent with inhibition of TDF hydrolysis. Additional studies are needed to determine the clinical relevance of this interaction.
© The Author(s) 2019. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Effects of sofosbuvir-based hepatitis C treatment on the pharmacokinetics of tenofovir in HIV/HCV-coinfected individuals receiving tenofovir disoproxil fumarate.J Antimicrob Chemother. 2018 Aug 1;73(8):2112-2119. doi: 10.1093/jac/dky146. J Antimicrob Chemother. 2018. PMID: 29746648 Free PMC article. Clinical Trial.
-
Incidence of Acute Kidney Injury in Patients Coinfected with HIV and Hepatitis C Virus Receiving Tenofovir Disoproxil Fumarate and Ledipasvir/Sofosbuvir in a Real-World, Urban, Ryan White Clinic.AIDS Res Hum Retroviruses. 2018 Aug;34(8):690-698. doi: 10.1089/AID.2017.0271. Epub 2018 Jun 19. AIDS Res Hum Retroviruses. 2018. PMID: 29766745 Free PMC article.
-
Nephrotoxicity Associated with Concomitant Use of Ledipasvir-Sofosbuvir and Tenofovir in a Patient with Hepatitis C Virus and Human Immunodeficiency Virus Coinfection.Pharmacotherapy. 2016 Sep;36(9):e148-53. doi: 10.1002/phar.1803. Pharmacotherapy. 2016. PMID: 27459733
-
Ledipasvir/Sofosbuvir: a review of its use in chronic hepatitis C.Drugs. 2015 Apr;75(6):675-85. doi: 10.1007/s40265-015-0381-2. Drugs. 2015. PMID: 25837989 Review.
-
Ledipasvir/sofosbuvir fixed-dose combination for treatment of hepatitis C virus genotype 4 infection.Drugs Today (Barc). 2016 Feb;52(2):111-7. doi: 10.1358/dot.2016.52.2.2449840. Drugs Today (Barc). 2016. PMID: 27092340 Review.
Cited by
-
Pharmacokinetics and renal safety of tenofovir alafenamide with boosted protease inhibitors and ledipasvir/sofosbuvir.J Antimicrob Chemother. 2020 Nov 1;75(11):3303-3310. doi: 10.1093/jac/dkaa299. J Antimicrob Chemother. 2020. PMID: 32766700 Free PMC article.
-
Real-world effectiveness of direct-acting antivirals in people living with human immunodeficiency virus and hepatitis C virus genotype 6 infections.World J Gastroenterol. 2022 Mar 21;28(11):1172-1183. doi: 10.3748/wjg.v28.i11.1172. World J Gastroenterol. 2022. PMID: 35431505 Free PMC article.
-
Determinants of adherence to daily PrEP measured as intracellular tenofovir diphosphate concentrations over 24 months of follow-up among men who have sex with men.Sex Transm Infect. 2023 Aug;99(5):303-310. doi: 10.1136/sextrans-2022-055499. Epub 2022 Sep 5. Sex Transm Infect. 2023. PMID: 37258273 Free PMC article.
-
Recommendations for interpreting intraerythrocytic tenofovir-diphosphate and emtricitabine-triphosphate for PrEP adherence assessment.J Acquir Immune Defic Syndr. 2025 Jun 19:10.1097/QAI.0000000000003715. doi: 10.1097/QAI.0000000000003715. Online ahead of print. J Acquir Immune Defic Syndr. 2025. PMID: 40536250 Free PMC article.
-
Investigation of the effect of verapamil on the regional absorption of sofosbuvir from rabbit intestine in situ.Daru. 2022 Jun;30(1):49-58. doi: 10.1007/s40199-021-00429-1. Epub 2022 Jan 13. Daru. 2022. PMID: 35023081 Free PMC article.
References
-
- Platt L, Easterbrook P, Gower E. et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 797–808. - PubMed
-
- AASLD-IDSA. Unique Populations. Patients With HIV/HCV Coinfection http://www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care.

