Plant-necrotroph co-transcriptome networks illuminate a metabolic battlefield
- PMID: 31081752
- PMCID: PMC6557632
- DOI: 10.7554/eLife.44279
Plant-necrotroph co-transcriptome networks illuminate a metabolic battlefield
Abstract
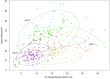
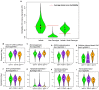
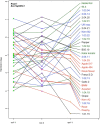
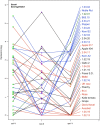
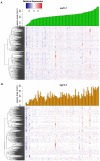
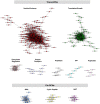
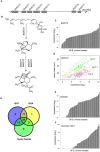
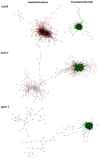
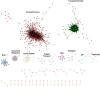
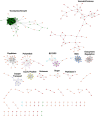
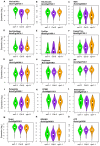
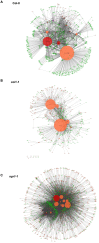
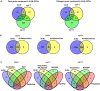
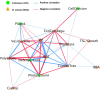
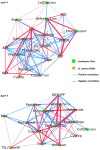
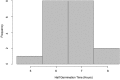
A central goal of studying host-pathogen interaction is to understand how host and pathogen manipulate each other to promote their own fitness in a pathosystem. Co-transcriptomic approaches can simultaneously analyze dual transcriptomes during infection and provide a systematic map of the cross-kingdom communication between two species. Here we used the Arabidopsis-B. cinerea pathosystem to test how plant host and fungal pathogen interact at the transcriptomic level. We assessed the impact of genetic diversity in pathogen and host by utilization of a collection of 96 isolates infection on Arabidopsis wild-type and two mutants with jasmonate or salicylic acid compromised immunities. We identified ten B. cinereagene co-expression networks (GCNs) that encode known or novel virulence mechanisms. Construction of a dual interaction network by combining four host- and ten pathogen-GCNs revealed potential connections between the fungal and plant GCNs. These co-transcriptome data shed lights on the potential mechanisms underlying host-pathogen interaction.
Keywords: A. thaliana; Arabidopsis; Botrytis cinerea; co-transcriptome; gene co-expression network; host-pathogen Interaction; pathosystem; plant biology.
© 2019, Zhang et al.
Conflict of interest statement
WZ, JC, DC, JF, RE, DC, SA No competing interests declared, DK Reviewing editor, eLife
Figures


References
-
- Arbelet D, Malfatti P, Simond-Côte E, Fontaine T, Desquilbet L, Expert D, Kunz C, Soulié MC. Disruption of the Bcchs3a chitin synthase gene in Botrytis cinerea is responsible for altered adhesion and overstimulation of host plant immunity. Molecular Plant-Microbe Interactions. 2010;23:1324–1334. doi: 10.1094/MPMI-02-10-0046. - DOI - PubMed
-
- Ascari J, Boaventura MA, Takahashi JA, Durán-Patrón R, Hernández-Galán R, Macías-Sánchez AJ, Collado IG. Phytotoxic activity and metabolism of botrytis cinerea and structure-activity relationships of isocaryolane derivatives. Journal of Natural Products. 2013;76:1016–1024. doi: 10.1021/np3009013. - DOI - PubMed
-
- Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, Molina A, Schulze-Lefert P. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science. 2009;323:101–106. doi: 10.1126/science.1163732. - DOI - PubMed

