Nicotine excites VIP interneurons to disinhibit pyramidal neurons in auditory cortex
- PMID: 31081950
- PMCID: PMC6767604
- DOI: 10.1002/syn.22116
Nicotine excites VIP interneurons to disinhibit pyramidal neurons in auditory cortex
Abstract
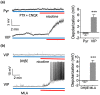
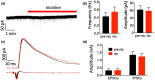
Nicotine activates nicotinic acetylcholine receptors and improves cognitive and sensory function, in part by its actions in cortical regions. Physiological studies show that nicotine amplifies stimulus-evoked responses in sensory cortex, potentially contributing to enhancement of sensory processing. However, the role of specific cell types and circuits in the nicotinic modulation of sensory cortex remains unclear. Here, we performed whole-cell recordings from pyramidal (Pyr) neurons and inhibitory interneurons expressing parvalbumin (PV), somatostatin (SOM), and vasoactive intestinal peptide (VIP) in mouse auditory cortex, in vitro. Bath application of nicotine strongly depolarized and excited VIP neurons, weakly depolarized Pyr neurons, and had no effect on the membrane potential of SOM or PV neurons. The use of receptor antagonists showed that nicotine's effects on VIP and Pyr neurons were direct and indirect, respectively. Nicotine also enhanced the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) in Pyr, VIP, and SOM, but not PV, cells. Using Designer Receptors Exclusively Activated by Designer Drugs (DREADDs), we show that chemogenetic inhibition of VIP neurons prevents nicotine's effects on Pyr neurons. Since VIP cells preferentially contact other inhibitory interneurons, we suggest that nicotine drives VIP cell firing to disinhibit Pyr cell somata, potentially making Pyr cells more responsive to auditory stimuli. In parallel, activation of VIP cells also directly inhibits Pyr neurons, likely altering integration of other synaptic inputs. These cellular and synaptic mechanisms likely contribute to nicotine's beneficial effects on cognitive and sensory function.
Keywords: VIP; interneuron; nicotine; nicotinic acetylcholine receptor; parvalbumin; pyramidal neuron; somatostatin.
© 2019 The Authors. Synapse Published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no competing financial interests. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
