Placebo-controlled randomized clinical trial testing the efficacy and safety of varenicline for smokers with HIV
- PMID: 31082665
- PMCID: PMC6588414
- DOI: 10.1016/j.drugalcdep.2019.03.011
Placebo-controlled randomized clinical trial testing the efficacy and safety of varenicline for smokers with HIV
Abstract
Background: People living with HIV/AIDS (PLWH) smoke tobacco at higher rates and have more difficulty quitting than the general population, which contributes to significant life-years lost. The effectiveness of varenicline, one of the most effective tobacco dependence treatments, is understudied in HIV. We evaluated the safety and efficacy of varenicline for smoking cessation among PLWH.
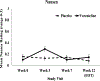
Methods: This was a single-site randomized, double-blind, placebo-controlled, phase 3 clinical trial (NCT01710137). PLWH on antiretroviral therapy (ART) who were treatment-seeking daily smokers were randomized (1:1) to 12 weeks of varenicline (n = 89) or placebo (n = 90). All participants were offered six smoking cessation behavioral counseling sessions. The primary outcome was 7-day point prevalence abstinence, confirmed with breath carbon monoxide, at Weeks 12 and 24. Continuous abstinence and time to relapse were secondary outcomes. Safety measures were treatment-related side effects, adverse events, blood pressure, viral load, and ART adherence.
Results: Of the 179 smokers, 81% were African American, and 68% were male. Varenicline increased cessation at Week 12 (28.1% vs. 12.1%; OR = 4.54, 95% CI:1.83-11.25, P = .001). Continuous abstinence from Week 9 to 12 was higher for varenicline vs. placebo (23.6% vs. 10%; OR = 4.65, 95% CI:1.71-12.67, P = .003); at Week 24, there was no effect of varenicline for point prevalence (14.6% vs. 10%), continuous abstinence (10.1% vs. 6.7%), or time to relapse (Ps > .05). There were no differences between varenicline and placebo on safety measures (Ps > .05).
Conclusions: Varenicline is safe and efficacious for short-term smoking cessation among PLWH and should be used to reduce tobacco-related life-years lost in this population.
Keywords: AIDS; HIV; Nicotine dependence; Smoking cessation; Tobacco use; Varenicline.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of Interest
Dr. Schnoll received medication and placebo free of charge from Pfizer for clinical trials and has provided consultation to Pfizer, GlaxoSmithKline, and Curaleaf. Dr. Gross serves on a Pfizer Data and Safety Monitoring Board for a drug unrelated to smoking or HIV. Dr. Ashare has an investigator-initiated grant from Novo Nordisk for a drug unrelated to the current study.
Figures


References
-
- U.S. Department of Health and Human Services. AIDS.gov: HIV and smoking U.S. Department of Health and Human Services, Washington, D.C.
-
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE, 2016. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double-blind, randomised, placebo-controlled clinical trial. Lancet 387, 2507–2520. - PubMed
-
- Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, Hansson A, Lichtenstein E, Henningfield J, Tsoh J, Hurt RD, Velicer W, 2002. Biochemical verification of tobacco use and cessation. Nicotine Tob. Res 4, 149–159.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

