Brain networks and their relevance for stroke rehabilitation
- PMID: 31082786
- PMCID: PMC6603430
- DOI: 10.1016/j.clinph.2019.04.004
Brain networks and their relevance for stroke rehabilitation
Abstract
Stroke has long been regarded as focal disease with circumscribed damage leading to neurological deficits. However, advances in methods for assessing the human brain and in statistics have enabled new tools for the examination of the consequences of stroke on brain structure and function. Thereby, it has become evident that stroke has impact on the entire brain and its network properties and can therefore be considered as a network disease. The present review first gives an overview of current methodological opportunities and pitfalls for assessing stroke-induced changes and reorganization in the human brain. We then summarize principles of plasticity after stroke that have emerged from the assessment of networks. Thereby, it is shown that neurological deficits do not only arise from focal tissue damage but also from local and remote changes in white-matter tracts and in neural interactions among wide-spread networks. Similarly, plasticity and clinical improvements are associated with specific compensatory structural and functional patterns of neural network interactions. Innovative treatment approaches have started to target such network patterns to enhance recovery. Network assessments to predict treatment response and to individualize rehabilitation is a promising way to enhance specific treatment effects and overall outcome after stroke.
Keywords: Network; Plasticity; Rehabilitation; Stroke.
Copyright © 2019 International Federation of Clinical Neurophysiology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare no conflicts of interest.
Figures
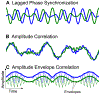


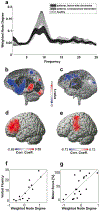
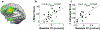
References
-
- Agarwal S, Sair HI, Pillai JJ. The Resting-State Functional Magnetic Resonance Imaging Regional Homogeneity Metrics-Kendall's Coefficient of Concordance-Regional Homogeneity and Coherence-Regional Homogeneity-Are Valid Indicators of Tumor-Related Neurovascular Uncoupling. Brain Connect. 2017;7:228–35. doi: 10.1089/brain.2016.0482. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

