Identification of miRNAs and Their Response to Cold Stress in Astragalus Membranaceus
- PMID: 31083391
- PMCID: PMC6572118
- DOI: 10.3390/biom9050182
Identification of miRNAs and Their Response to Cold Stress in Astragalus Membranaceus
Abstract
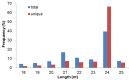
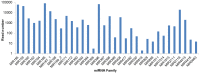
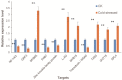
Astragalus membranaceus is an important medicinal plant widely cultivated in East Asia. MicroRNAs (miRNAs) are endogenous regulatory molecules that play essential roles in plant growth, development, and the response to environmental stresses. Cold is one of the key environmental factors affecting the yield and quality of A. membranaceus, and miRNAs may mediate the gene regulation network under cold stress in A. membranaceus. To identify miRNAs and reveal their functions in cold stress response in A. membranaceus, small RNA sequencing was conducted followed by bioinformatics analysis, and quantitative real time PCR (qRT-PCR) analysis was performed to profile the expression of miRNAs under cold stress. A total of 168 conserved miRNAs belonging to 34 families and 14 putative non-conserved miRNAs were identified. Many miRNA targets were predicted and these targets were involved in diversified regulatory and metabolic pathways. By using qRT-PCR, 27 miRNAs were found to be responsive to cold stress, including 4 cold stress-induced and 17 cold-repressed conserved miRNAs, and 6 cold-induced non-conserved miRNAs. These cold-responsive miRNAs probably mediate the response to cold stress by regulating development, hormone signaling, defense, redox homeostasis, and secondary metabolism in A. membranaceus. These cold-corresponsive miRNAs may be used as the candidate genes in further molecular breeding for improving cold tolerance of A. membranaceus.
Keywords: Astragalus membranaceus; cold stress; miR390; miRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Nalbantsoy A., Nesil T., Yilmaz-Dilsiz O., Aksu G., Khan S., Bedir E. Evaluation of the immunomodulatory properties in mice and in vitro anti-inflammatory activity of cycloartane type saponins from Astragalus species. J. Ethnopharmacol. 2012;139:574–581. doi: 10.1016/j.jep.2011.11.053. - DOI - PubMed
-
- Leung W.C., Leung K.N. In vitro and in vivo anti-tumor effects of Astragalus membranaceus. Cancer Lett. 2007;252:43–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

