Multiple Glycation Sites in Blood Plasma Proteins as an Integrated Biomarker of Type 2 Diabetes Mellitus
- PMID: 31083443
- PMCID: PMC6539793
- DOI: 10.3390/ijms20092329
Multiple Glycation Sites in Blood Plasma Proteins as an Integrated Biomarker of Type 2 Diabetes Mellitus
Abstract
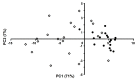
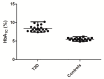
Type 2 diabetes mellitus (T2DM) is one of the most widely spread metabolic diseases. Because of its asymptomatic onset and slow development, early diagnosis and adequate glycaemic control are the prerequisites for successful T2DM therapy. In this context, individual amino acid residues might be sensitive indicators of alterations in blood glycation levels. Moreover, due to a large variation in the half-life times of plasma proteins, a generalized biomarker, based on multiple glycation sites, might provide comprehensive control of the glycemic status across any desired time span. Therefore, here, we address the patterns of glycation sites in highly-abundant blood plasma proteins of T2DM patients and corresponding age- and gender-matched controls by comprehensive liquid chromatography-mass spectrometry (LC-MS). The analysis revealed 42 lysyl residues, significantly upregulated under hyperglycemic conditions. Thereby, for 32 glycation sites, biomarker behavior was demonstrated here for the first time. The differentially glycated lysines represented nine plasma proteins with half-lives from 2 to 21 days, giving access to an integrated biomarker based on multiple protein-specific Amadori peptides. The validation of this biomarker relied on linear discriminant analysis (LDA) with random sub-sampling of the training set and leave-one-out cross-validation (LOOCV), which resulted in an accuracy, specificity, and sensitivity of 92%, 100%, and 85%, respectively.
Keywords: Amadori compounds; biomarkers; glycation; glycation sites; label-free quantification; linear discriminant analysis; mass spectrometry; plasma proteins; type 2 diabetes mellitus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

