Seven new Drimane-Type Sesquiterpenoids from a Marine-Derived Fungus Paraconiothyrium sporulosum YK-03
- PMID: 31083454
- PMCID: PMC6539489
- DOI: 10.3390/molecules24091817
Seven new Drimane-Type Sesquiterpenoids from a Marine-Derived Fungus Paraconiothyrium sporulosum YK-03
Abstract
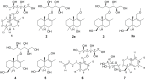
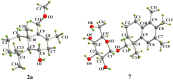
Seven new drimane-type sesquiterpenoids, namely the sporulositols A-D (1-4), 6-hydroxydiaporol (5), seco-sporulositol (6) and sporuloside (7) were isolated from the ethyl acetate extract of fermentation broth for a marine-derived fungus Paraconiothyrium sporulosum YK-03. Their structures were elucidated by analysis of extensive spectroscopic data, and the absolute configurations were established by crystal X-ray diffraction analysis and comparisons of circular dichroism data. Among them, sporulositols A-E (1-4) and seco-sporulositol (6) represent the first five examples of a unique class of drimanic mannitol derivatives, while compounds 6 and 7 may represent two new series of natural drimanes, possessing an aromatic ring with a rare 4,5-secodrimanic skeleton and an unusual CH3-15 rearranged drimanic α-D-glucopyranside, respectively. Furthermore, the origin of mannitol moiety was investigated by reliable HPLC and NMR analyses.
Keywords: Paraconiothyrium; Paraconiothyrium sporulosum; drimane-type sesquiterpenoid; seco-sporulositol; sporuloside; sporulositol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Verkley G.J.M., Silva M. d., Wicklow D.T., Crou P.W. Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Stud. Mycol. 2004;50:323–335.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

