Flavivirus Replication Organelle Biogenesis in the Endoplasmic Reticulum: Comparison with Other Single-Stranded Positive-Sense RNA Viruses
- PMID: 31083507
- PMCID: PMC6539296
- DOI: 10.3390/ijms20092336
Flavivirus Replication Organelle Biogenesis in the Endoplasmic Reticulum: Comparison with Other Single-Stranded Positive-Sense RNA Viruses
Abstract
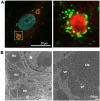
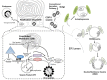
Some single-stranded positive-sense RNA [ssRNA(+)] viruses, including Flavivirus, generate specific organelle-like structures in the host endoplasmic reticulum (ER). These structures are called virus replication organelles and consist of two distinct subdomains, the vesicle packets (VPs) and the convoluted membranes (CMs). The VPs are clusters of small vesicle compartments and are considered to be the site of viral genome replication. The CMs are electron-dense amorphous structures observed in proximity to the VPs, but the exact roles of CMs are mostly unknown. Several recent studies have revealed that flaviviruses recruit several host factors that are usually used for the biogenesis of other conventional organelles and usurp their function to generate virus replication organelles. In the current review, we summarize recent studies focusing on the role of host factors in the formation of virus replication organelles and discuss how these intricate membrane structures are organized.
Keywords: ESCRT; autophagy; endoplasmic reticulum; flavivirus; phosphatidylinositol; reticulons.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

