Ethylene Glycol Dicyclopentenyl (Meth)Acrylate Homo and Block Copolymers via Nitroxide Mediated Polymerization
- PMID: 31083510
- PMCID: PMC6539251
- DOI: 10.3390/ma12091547
Ethylene Glycol Dicyclopentenyl (Meth)Acrylate Homo and Block Copolymers via Nitroxide Mediated Polymerization
Abstract
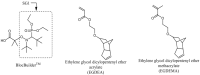
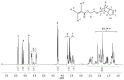
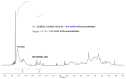
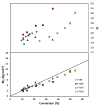
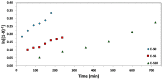
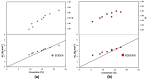
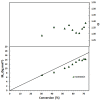
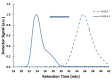
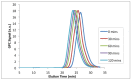
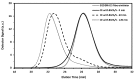
Nitroxide-mediated polymerization (NMP), (homo and block copolymerization with styrene (S) and butyl methacrylate/S) of ethylene glycol dicyclopentenyl ether (meth)acrylates (EGDEA and EGDEMA) was studied using BlocBuilder alkoxyamines. EGDEA homopolymerization was not well-controlled, independent of temperature (90-120 °C), or additional free nitroxide (0-10 mol%) used. Number average molecular weights (Mn) achieved for poly(EGDEA) were 4.0-9.5 kg mol-1 and were accompanied by high dispersity (Ð = Mw/Mn = 1.62-2.09). Re-initiation and chain extension of the poly(EGDEA) chains with styrene (S) indicated some block copolymer formation, but a high fraction of chains were terminated irreversibly. EGDEA-stat-S statistical copolymerizations with a low mol fraction S in initial feed, fS,0 = 0.05, were slightly better controlled compared to poly(EGDEA) homopolymerizations (Ð was reduced to 1.44 compared to 1.62 at similar conditions). EGDEMA, in contrast, was successfully polymerized using a small fraction of S (fS,0 ~ 10 mol%) to high conversion (72%) to form well-defined EGDEMA-rich random copolymer (molar composition = FEGDEMA = 0.87) of Mn = 14.3 kg mol-1 and Ð = 1.38. EGDEMA-rich compositions were also polymerized with the unimolecular succinimidyl ester form of BlocBuilder initiator, NHS-BlocBuilder with similar results, although Ðs were higher ~1.6. Chain extensions resulted in monomodal shifts to higher molecular weights, indicating good chain end fidelity.
Keywords: block copolymers; copolymerization; nitroxide mediated polymerization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Eisenhart E.K., Bowe M.D., Weir W.D., Wolfersberger M.A.H. Reactive Coalescents. 5,349,026. U.S. Patent. 1998 Sep 20;
-
- Speece D.G., Jr., Weir W.D., Eisenhart E.K., Bowe M.D., Wolfersberger M.A.H. Reactive Coalescents. 6,451,380. U.S. Patent. 2002 Sep 17;
-
- Adlington K., Nguyen N.T., Eaves E., Yang J., Chang C.Y., Li J., Gower A.L., Stimpson A., Anderson D.G., Langer R., et al. Application of targeted molecular and material property optimization to bacterial attachment-resistant (Meth) acrylate polymers. Biomacromolecules. 2016;17:2830–2838. doi: 10.1021/acs.biomac.6b00615. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

