A Role for NF-κB in Organ Specific Cancer and Cancer Stem Cells
- PMID: 31083587
- PMCID: PMC6563002
- DOI: 10.3390/cancers11050655
A Role for NF-κB in Organ Specific Cancer and Cancer Stem Cells
Abstract
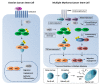
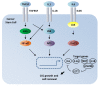
Cancer stem cells (CSCs) account for tumor initiation, invasiveness, metastasis, and recurrence in a broad range of human cancers. Although being a key player in cancer development and progression by stimulating proliferation and metastasis and preventing apoptosis, the role of the transcription factor NF-κB in cancer stem cells is still underestimated. In the present review, we will evaluate the role of NF-κB in CSCs of glioblastoma multiforme, ovarian cancer, multiple myeloma, lung cancer, colon cancer, prostate cancer, as well as cancer of the bone. Next to summarizing current knowledge regarding the presence and contribution of CSCs to the respective types of cancer, we will emphasize NF-κB-mediated signaling pathways directly involved in maintaining characteristics of cancer stem cells associated to tumor progression. Here, we will also focus on the status of NF-κB-activity predominantly in CSC populations and the tumor mass. Genetic alterations leading to NF-κB activity in glioblastoma, ependymoma, and multiple myeloma will be discussed.
Keywords: NF-κB; bone cancer; cancer stem cells; colon cancer; glioblastoma multiforme; lung cancer; multiple myeloma; ovarian cancer; pediatric cancer; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Singh S.K., Clarke I.D., Terasaki M., Bonn V.E., Hawkins C., Squire J., Dirks P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. - PubMed
-
- Di Tomaso T., Mazzoleni S., Wang E., Sovena G., Clavenna D., Franzin A., Mortini P., Ferrone S., Doglioni C., Marincola F.M., et al. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin. Cancer Res. 2010;16:800–813. doi: 10.1158/1078-0432.CCR-09-2730. - DOI - PMC - PubMed
-
- Caussinus E., Hirth F. Asymmetric Cell Division. Volume 45. Springer; Berlin/Heidelberg, Germany: 2007. Asymmetric stem cell division in development and cancer; pp. 205–225. Progress in Molecular and Subcellular Biology. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

