Aerobic Exercise Training Decreases Hepatic Asprosin in Diabetic Rats
- PMID: 31083617
- PMCID: PMC6572469
- DOI: 10.3390/jcm8050666
Aerobic Exercise Training Decreases Hepatic Asprosin in Diabetic Rats
Abstract
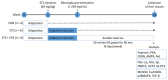
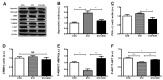
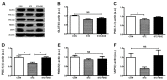
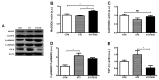
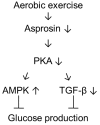
Asprosin, a novel hormone released from white adipose tissue, regulates hepatic glucose metabolism and is pathologically elevated in the presence of insulin resistance. It is unknown whether aerobic exercise training affects asprosin levels in type 1 diabetes mellitus (T1DM). The aim of this study was to determine whether (1) aerobic exercise training could decrease asprosin levels in the liver of streptozotocin (STZ)-induced diabetic rats and (2) the reduction in asprosin levels could induce asprosin-dependent downstream pathways. Five-week-old male Sprague-Dawley rats were randomly divided into control, STZ-induced diabetes (STZ), and STZ with aerobic exercise training groups (n = 6/group). T1DM was induced by a single dose of STZ (65 mg/kg intraperitoneally (i.p.)). The exercise group was made to run on a treadmill for 60 min at a speed of 20 m/min, 4 days per week for 8 weeks. Aerobic exercise training reduced the protein levels of asprosin, PKA, and TGF-β but increased those of AMPK, Akt, PGC-1β, and MnSOD. These results suggest that aerobic exercise training affects hepatic asprosin-dependent PKA/TGF-β and AMPK downstream pathways in T1DM.
Keywords: AMPK; PKA; TGF-β; aerobic exercise; asprosin; liver; type 1 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

