Understanding Cell Penetration of Cyclic Peptides
- PMID: 31083977
- PMCID: PMC6739158
- DOI: 10.1021/acs.chemrev.9b00008
Understanding Cell Penetration of Cyclic Peptides
Abstract
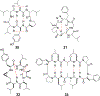
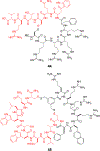
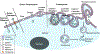
Approximately 75% of all disease-relevant human proteins, including those involved in intracellular protein-protein interactions (PPIs), are undruggable with the current drug modalities (i.e., small molecules and biologics). Macrocyclic peptides provide a potential solution to these undruggable targets because their larger sizes (relative to conventional small molecules) endow them the capability of binding to flat PPI interfaces with antibody-like affinity and specificity. Powerful combinatorial library technologies have been developed to routinely identify cyclic peptides as potent, specific inhibitors against proteins including PPI targets. However, with the exception of a very small set of sequences, the vast majority of cyclic peptides are impermeable to the cell membrane, preventing their application against intracellular targets. This Review examines common structural features that render most cyclic peptides membrane impermeable, as well as the unique features that allow the minority of sequences to enter the cell interior by passive diffusion, endocytosis/endosomal escape, or other mechanisms. We also present the current state of knowledge about the molecular mechanisms of cell penetration, the various strategies for designing cell-permeable, biologically active cyclic peptides against intracellular targets, and the assay methods available to quantify their cell-permeability.
Figures

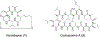


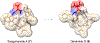



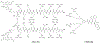










References
-
- Verdine GL; Walensky LD The Challenge of Drugging Undruggable Targets in Cancer: Lessons Learned from Targeting BCL-2 Family Members. Clin. Cancer Res. 2007, 13, 7264–7270. - PubMed
-
- Gentilucci L; De Marco R; Cerisoli L Chemical Modifications Designed to Improve Peptide Stability: Incorporation of Non-Natural Amino Acids, Psuedo-Peptide Bonds, and Cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203. - PubMed
-
- Chang YS; Graves B; Guerlavais V; Tovar C; Packman K; To K; Olson KA; Kesavan K; Gangurde P; Mukherjee A; et al. Stapled α-Helical Peptide Drug Development: A Potent Dual Inhibitor of MDM2 and MDMX for p53-Dependent Cancer Therapy. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 3445–3454. - PMC - PubMed
-
- Morimoto J; Hayashi Y; Suga H Discovery of Macrocyclic Peptides Armed with a Mechanism-Based Warhead: Isoform-Selective Inhibition of Human Deacetylase SIRT2. Angew. Chem. Int. Ed. Engl. 2012, 51, 3423–3427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

