Use of Medicare Claims to Identify Adverse Clinical Outcomes After Mitral Valve Repair
- PMID: 31084236
- PMCID: PMC6760250
- DOI: 10.1161/CIRCINTERVENTIONS.118.007451
Use of Medicare Claims to Identify Adverse Clinical Outcomes After Mitral Valve Repair
Abstract
Background: Clinical event committees are commonly employed for event validation in clinical studies, but little is known about the comparative performance of administrative claims data versus clinician-triggered event adjudication for ascertainment of adverse events in structural heart disease studies.
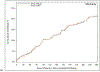
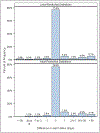
Methods and results: Medicare claims were linked to 418 patients >65 years of age who underwent transcatheter mitral valve repair (MitraClip) for severe mitral regurgitation from 2007 to 2013 as part of the EVEREST II (Endovascular Valve Edge-to-Edge Repair Study II) High-Risk Registry or the REALISM (Real World Expanded Multicenter Study of the MitraClip System) Continued-Access Registry. Each registry adjudicated mortality, heart failure hospitalization, renal failure, ventilation, and bleeding/transfusion within 1 year. Concordance of claims-based outcomes with events was assessed in 3 ways: 1-year occurrence, cumulative incidence, and synchrony of first events. For event occurrence, positive predictive value (PPV) of claims versus adjudication was the highest for mortality (PPV=97%) and heart failure hospitalization (PPV=69%) but lower for bleeding (PPV=40%) and renal failure (PPV=19%). Whereas claims-based cumulative incidence for mortality, heart failure hospitalization, and renal failure were consistent with clinician-triggered adjudication, incidence curves for bleeding events and ventilation diverged, with claims identifying a greater number of events. When events were detected by both methods, however, over 75% of event dates matched exactly. Mitral valve reinterventions were identified through claims with perfect sensitivity and specificity relative to physician adjudication.
Conclusions: Ascertainment of mortality, heart failure hospitalization, and renal failure was highly concordant between physician adjudication and administrative claims. Further work is necessary to determine the role of administrative claims in event ascertainment in both prospective and retrospective studies of structural heart disease.
Keywords: heart failure; humans; mitral valve; mitral valve insufficiency; registries.
Figures





Comment in
-
Putting Theory to the Test.Circ Cardiovasc Interv. 2019 May;12(5):e007953. doi: 10.1161/CIRCINTERVENTIONS.119.007953. Circ Cardiovasc Interv. 2019. PMID: 31084240 Free PMC article. No abstract available.
References
-
- Sedrakyan A, Marinac-Dabic D, Normand SL, Mushlin A and Gross T. A framework for evidence evaluation and methodological issues in implantable device studies. Med Care. 2010;48:S121–8. - PubMed
-
- Granger BB. Practical steps for evidence-based practice: putting one foot in front of the other. AACN Adv Crit Care. 2008;19:314–24. - PubMed
-
- Stone GW, Adams DH, Abraham WT, Kappetein AP, Genereux P, Vranckx P, Mehran R, Kuck KH, Leon MB, Piazza N, Head SJ, Filippatos G, Vahanian AS and Mitral Valve Academic Research C. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2: Endpoint Definitions: A Consensus Document From the Mitral Valve Academic Research Consortium. J Am Coll Cardiol. 2015;66:308–321. - PubMed
-
- Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodes-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW and Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–54. - PubMed
-
- Draft Guidance: Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices; Draft Guidance for Industry and Food and Drug Administration Staff. Docket No. FDA-2016-D-2153. 2016.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

