Programming Structured DNA Assemblies to Probe Biophysical Processes
- PMID: 31084582
- PMCID: PMC7035826
- DOI: 10.1146/annurev-biophys-052118-115259
Programming Structured DNA Assemblies to Probe Biophysical Processes
Abstract
Structural DNA nanotechnology is beginning to emerge as a widely accessible research tool to mechanistically study diverse biophysical processes. Enabled by scaffolded DNA origami in which a long single strand of DNA is weaved throughout an entire target nucleic acid assembly to ensure its proper folding, assemblies of nearly any geometric shape can now be programmed in a fully automatic manner to interface with biology on the 1-100-nm scale. Here, we review the major design and synthesis principles that have enabled the fabrication of a specific subclass of scaffolded DNA origami objects called wireframe assemblies. These objects offer unprecedented control over the nanoscale organization of biomolecules, including biomolecular copy numbers, presentation on convex or concave geometries, and internal versus external functionalization, in addition to stability in physiological buffer. To highlight the power and versatility of this synthetic structural biology approach to probing molecular and cellular biophysics, we feature its application to three leading areas of investigation: light harvesting and nanoscale energy transport, RNA structural biology, and immune receptor signaling, with an outlook toward unique mechanistic insight that may be gained in these areas in the coming decade.
Keywords: DNA origami; RNA structural biology; computational design; immunology; light harvesting; nanoscale energy transport; nanotechnology; synthetic structural biology.
Figures


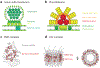
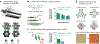


References
-
- Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801 - PubMed
-
- Albinsson B, Hannestad JK, Börjesson K. 2012. Functionalized DNA nanostructures for light harvesting and charge separation. Coord. Chem. Rev 256:2399–413
-
- Anderson RM, Kwon M, Strobel SA. 2007. Toward ribosomal RNA catalytic activity in the absence of protein. J. Mol. Evol 64:472–83 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

