Modeling Pneumonic Plague in Human Precision-Cut Lung Slices Highlights a Role for the Plasminogen Activator Protease in Facilitating Type 3 Secretion
- PMID: 31085709
- PMCID: PMC6652753
- DOI: 10.1128/IAI.00175-19
Modeling Pneumonic Plague in Human Precision-Cut Lung Slices Highlights a Role for the Plasminogen Activator Protease in Facilitating Type 3 Secretion
Abstract
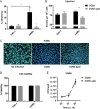
Pneumonic plague is the deadliest form of disease caused by Yersinia pestis Key to the progression of infection is the activity of the plasminogen activator protease Pla. Deletion of Pla results in a decreased Y. pestis bacterial burden in the lung and failure to progress into the lethal proinflammatory phase of disease. While a number of putative functions have been attributed to Pla, its precise role in the pathogenesis of pneumonic plague is yet to be defined. Here, we show that Pla facilitates type 3 secretion into primary alveolar macrophages but not into the commonly used THP-1 cell line. We also establish human precision-cut lung slices as a platform for modeling early host/pathogen interactions during pneumonic plague and solidify the role of Pla in promoting optimal type 3 secretion using primary human tissue with relevant host cell heterogeneity. These results position Pla as a key player in the early host/pathogen interactions that define pneumonic plague and showcase the utility of human precision-cut lung slices as a platform to evaluate pulmonary infection by bacterial pathogens.
Keywords: Pla; Yersinia; Yersinia pestis; hPCLS; human precision-cut lung slices; plague; plasminogen activator protease; pneumonic plague; pulmonary infection; type 3 secretion.
Copyright © 2019 Banerjee et al.
Figures



References
-
- Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Koerner JF, Layton M, McDade J, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Schoch-Spana M, Tonat K. 2000. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA 283:2281–2290. doi:10.1001/jama.283.17.2281. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

