Tart Cherry Concentrate Does Not Alter the Gut Microbiome, Glycaemic Control or Systemic Inflammation in a Middle-Aged Population
- PMID: 31085979
- PMCID: PMC6567170
- DOI: 10.3390/nu11051063
Tart Cherry Concentrate Does Not Alter the Gut Microbiome, Glycaemic Control or Systemic Inflammation in a Middle-Aged Population
Abstract
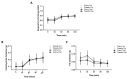
Limited evidence suggests that the consumption of polyphenols may improve glycaemic control and insulin sensitivity. The gut microbiome produces phenolic metabolites and increases their bioavailability. A handful of studies have suggested that polyphenol consumption alters gut microbiome composition. There are no data available investigating such effects in polyphenol-rich Montmorency cherry (MC) supplementation. A total of 28 participants (aged 40-60 years) were randomized to receive daily MC or glucose and energy-matched placebo supplementation for 4 wk. Faecal and blood samples were obtained at baseline and at 4 wk. There was no clear effect of supplementation on glucose handling (Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) and Gutt indices), although the Matsuda index decreased significantly in the MC group post-supplementation, reflecting an increase in serum insulin concentration. Contrastingly, placebo, but not MC supplementation induced a 6% increase in the Oral Glucose Insulin Sensitivity (OGIS) estimate of glucose clearance. Serum IL-6 and C reactive protein were unaltered by either supplement. The faecal bacterial microbiome was sequenced; species richness and diversity were unchanged by MC or placebo and no significant correlation existed between changes in Bacteroides and Faecalibacterium abundance and any index of insulin sensitivity. Therefore, 4 weeks of MC supplementation did not alter the gut microbiome, glycaemic control or systemic concentrations of IL-6 and CRP in a middle-aged population.
Keywords: Montmorency; cherry; microbiome; polyphenol.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo.J Nutr Biochem. 2018 Sep;59:160-172. doi: 10.1016/j.jnutbio.2018.04.001. Epub 2018 Apr 7. J Nutr Biochem. 2018. PMID: 30055451
-
Montmorency cherry supplementation attenuates vascular dysfunction induced by prolonged forearm occlusion in overweight, middle-aged men.J Appl Physiol (1985). 2019 Jan 1;126(1):246-254. doi: 10.1152/japplphysiol.00804.2018. Epub 2018 Nov 29. J Appl Physiol (1985). 2019. PMID: 30496705 Free PMC article. Clinical Trial.
-
Effect of tart cherry juice on recovery and next day performance in well-trained Water Polo players.J Int Soc Sports Nutr. 2016 Nov 14;13:41. doi: 10.1186/s12970-016-0151-x. eCollection 2016. J Int Soc Sports Nutr. 2016. PMID: 27895542 Free PMC article. Clinical Trial.
-
Tart Cherries and health: Current knowledge and need for a better understanding of the fate of phytochemicals in the human gastrointestinal tract.Crit Rev Food Sci Nutr. 2019;59(4):626-638. doi: 10.1080/10408398.2017.1384918. Epub 2017 Oct 30. Crit Rev Food Sci Nutr. 2019. PMID: 28956621 Review.
-
Tart Cherry Supplementation and Recovery From Strenuous Exercise: A Systematic Review and Meta-Analysis.Int J Sport Nutr Exerc Metab. 2021 Mar 1;31(2):154-167. doi: 10.1123/ijsnem.2020-0145. Epub 2021 Jan 13. Int J Sport Nutr Exerc Metab. 2021. PMID: 33440334
Cited by
-
Differentiation of Blastocystis and parasitic archamoebids encountered in untreated wastewater samples by amplicon-based next-generation sequencing.Parasite Epidemiol Control. 2019 Dec 21;9:e00131. doi: 10.1016/j.parepi.2019.e00131. eCollection 2020 May. Parasite Epidemiol Control. 2019. PMID: 31909230 Free PMC article.
-
A Polyphenol Enriched Variety of Apple Alters Circulating Immune Cell Gene Expression and Faecal Microbiota Composition in Healthy Adults: A Randomized Controlled Trial.Nutrients. 2021 Mar 27;13(4):1092. doi: 10.3390/nu13041092. Nutrients. 2021. PMID: 33801641 Free PMC article. Clinical Trial.
-
Lack of a Synergistic Effect on Cardiometabolic and Redox Markers in a Dietary Supplementation with Anthocyanins and Xanthophylls in Postmenopausal Women.Nutrients. 2019 Jul 5;11(7):1533. doi: 10.3390/nu11071533. Nutrients. 2019. PMID: 31284490 Free PMC article. Clinical Trial.
-
Effect of fruit intake on functional constipation: A systematic review and meta-analysis of randomized and crossover studies.Front Nutr. 2022 Oct 6;9:1018502. doi: 10.3389/fnut.2022.1018502. eCollection 2022. Front Nutr. 2022. PMID: 36276840 Free PMC article.
-
Review of Analytical Methods and Reporting of the Polyphenol Content of Tart Cherry Supplements in Human Supplementation Studies Investigating Health and Exercise Performance Effects: Recommendations for Good Practice.Front Nutr. 2021 Mar 26;8:652094. doi: 10.3389/fnut.2021.652094. eCollection 2021. Front Nutr. 2021. PMID: 33842524 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

