Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications
- PMID: 31085992
- PMCID: PMC6539070
- DOI: 10.3390/ijms20092358
Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications
Abstract
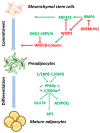
Obesity is a critical risk factor for the development of type 2 diabetes (T2D), and its prevalence is rising worldwide. White adipose tissue (WAT) has a crucial role in regulating systemic energy homeostasis. Adipose tissue expands by a combination of an increase in adipocyte size (hypertrophy) and number (hyperplasia). The recruitment and differentiation of adipose precursor cells in the subcutaneous adipose tissue (SAT), rather than merely inflating the cells, would be protective from the obesity-associated metabolic complications. In metabolically unhealthy obesity, the storage capacity of SAT, the largest WAT depot, is limited, and further caloric overload leads to the fat accumulation in ectopic tissues (e.g., liver, skeletal muscle, and heart) and in the visceral adipose depots, an event commonly defined as "lipotoxicity." Excessive ectopic lipid accumulation leads to local inflammation and insulin resistance (IR). Indeed, overnutrition triggers uncontrolled inflammatory responses in WAT, leading to chronic low-grade inflammation, therefore fostering the progression of IR. This review summarizes the current knowledge on WAT dysfunction in obesity and its associated metabolic abnormalities, such as IR. A better understanding of the mechanisms regulating adipose tissue expansion in obesity is required for the development of future therapeutic approaches in obesity-associated metabolic complications.
Keywords: adipogenesis; adipose tissue; adipose tissue dysfunction; diabetes; ectopic lipid deposition; hypertrophic obesity; inflammation; insulin resistance; lipotoxicity; obesity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

