Glycation of Plant Proteins: Regulatory Roles and Interplay with Sugar Signalling?
- PMID: 31086058
- PMCID: PMC6539852
- DOI: 10.3390/ijms20092366
Glycation of Plant Proteins: Regulatory Roles and Interplay with Sugar Signalling?
Abstract
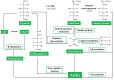
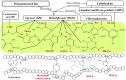
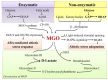
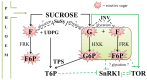
Glycation can be defined as an array of non-enzymatic post-translational modifications of proteins formed by their interaction with reducing carbohydrates and carbonyl products of their degradation. Initial steps of this process rely on reducing sugars and result in the formation of early glycation products-Amadori and Heyns compounds via Schiff base intermediates, whereas their oxidative degradation or reactions of proteins with α-dicarbonyl compounds yield a heterogeneous group of advanced glycation end products (AGEs). These compounds accompany thermal processing of protein-containing foods and are known to impact on ageing, pathogenesis of diabetes mellitus and Alzheimer's disease in mammals. Surprisingly, despite high tissue carbohydrate contents, glycation of plant proteins was addressed only recently and its physiological role in plants is still not understood. Therefore, here we summarize and critically discuss the first steps done in the field of plant protein glycation during the last decade. We consider the main features of plant glycated proteome and discuss them in the context of characteristic metabolic background. Further, we address the possible role of protein glycation in plants and consider its probable contribution to protein degradation, methylglyoxal and sugar signalling, as well as interplay with antioxidant defense.
Keywords: advanced glycation end products (AGEs); methylglyoxal; plant glycation; protein degradation; protein glycation; sugar signalling; thermal processing of foods.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

