Diverse compounds from pleuromutilin lead to a thioredoxin inhibitor and inducer of ferroptosis
- PMID: 31086302
- PMCID: PMC6639018
- DOI: 10.1038/s41557-019-0261-6
Diverse compounds from pleuromutilin lead to a thioredoxin inhibitor and inducer of ferroptosis
Abstract
The chemical diversification of natural products provides a robust and general method for the creation of stereochemically rich and structurally diverse small molecules. The resulting compounds have physicochemical traits different from those in most screening collections, and as such are an excellent source for biological discovery. Herein, we subject the diterpene natural product pleuromutilin to reaction sequences focused on creating ring system diversity in few synthetic steps. This effort resulted in a collection of compounds with previously unreported ring systems, providing a novel set of structurally diverse and highly complex compounds suitable for screening in a variety of different settings. Biological evaluation identified the novel compound ferroptocide, a small molecule that rapidly and robustly induces ferroptotic death of cancer cells. Target identification efforts and CRISPR knockout studies reveal that ferroptocide is an inhibitor of thioredoxin, a key component of the antioxidant system in the cell. Ferroptocide positively modulates the immune system in a murine model of breast cancer and will be a useful tool to study the utility of pro-ferroptotic agents for treatment of cancer.
Conflict of interest statement
Competing interests
The University of Illinois has filed patents on some compounds described in this manuscript.
Figures

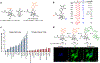
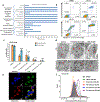


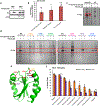
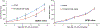
Comment in
-
Diverse engineering.Nat Chem. 2019 Jun;11(6):499-500. doi: 10.1038/s41557-019-0269-y. Nat Chem. 2019. PMID: 31086301 No abstract available.
References
-
- Lovering F, Bikker J & Humblet C Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem 52, 6752–6756, (2009). - PubMed
-
- Swinney DC & Anthony J How were new medicines discovered? Nat. Rev. Drug Discov 10, 507–519, (2011). - PubMed
-
- Galloway WRJD, Isidro-Llobet A & Spring DR Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun 1, 80–93, (2010). - PubMed
-
- Morrison KC & Hergenrother PJ Natural products as starting points for the synthesis of complex and diverse compounds. Nat. Prod. Rep 31, 6–14, (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

