Bacillus subtilis cell diameter is determined by the opposing actions of two distinct cell wall synthetic systems
- PMID: 31086310
- PMCID: PMC6656618
- DOI: 10.1038/s41564-019-0439-0
Bacillus subtilis cell diameter is determined by the opposing actions of two distinct cell wall synthetic systems
Abstract
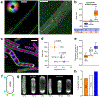
Rod-shaped bacteria grow by adding material into their cell wall via the action of two spatially distinct enzymatic systems: the Rod complex moves around the cell circumference, whereas class A penicillin-binding proteins (aPBPs) do not. To understand how the combined action of these two systems defines bacterial dimensions, we examined how each affects the growth and width of Bacillus subtilis as well as the mechanical anisotropy and orientation of material within their sacculi. Rod width is not determined by MreB, rather it depends on the balance between the systems: the Rod complex reduces diameter, whereas aPBPs increase it. Increased Rod-complex activity correlates with an increased density of directional MreB filaments and a greater fraction of directional PBP2a enzymes. This increased circumferential synthesis increases the relative quantity of oriented material within the sacculi, making them more resistant to stretching across their width, thereby reinforcing rod shape. Together, these experiments explain how the combined action of the two main cell wall synthetic systems builds and maintains rods of different widths. Escherichia coli Rod mutants also show the same correlation between width and directional MreB filament density, suggesting this model may be generalizable to bacteria that elongate via the Rod complex.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

