A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip
- PMID: 31086325
- PMCID: PMC6658209
- DOI: 10.1038/s41551-019-0397-0
A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip
Erratum in
-
Author Correction: A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip.Nat Biomed Eng. 2019 Jul;3(7):583. doi: 10.1038/s41551-019-0428-x. Nat Biomed Eng. 2019. PMID: 31213704 Free PMC article.
Abstract
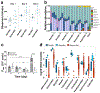
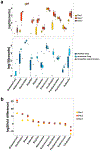
The diverse bacterial populations that comprise the commensal microbiome of the human intestine play a central role in health and disease. A method that sustains complex microbial communities in direct contact with living human intestinal cells and their overlying mucus layer in vitro would thus enable the investigation of host-microbiome interactions. Here, we show the extended coculture of living human intestinal epithelium with stable communities of aerobic and anaerobic human gut microbiota, using a microfluidic intestine-on-a-chip that permits the control and real-time assessment of physiologically relevant oxygen gradients. When compared to aerobic coculture conditions, the establishment of a transluminal hypoxia gradient in the chip increased intestinal barrier function and sustained a physiologically relevant level of microbial diversity, consisting of over 200 unique operational taxonomic units from 11 different genera and an abundance of obligate anaerobic bacteria, with ratios of Firmicutes and Bacteroidetes similar to those observed in human faeces. The intestine-on-a-chip may serve as a discovery tool for the development of microbiome-related therapeutics, probiotics and nutraceuticals.
Figures



Comment in
-
Anaerobic intestine-on-a-chip system enables complex microbiota co-culture.Nat Rev Gastroenterol Hepatol. 2019 Jul;16(7):390. doi: 10.1038/s41575-019-0162-6. Nat Rev Gastroenterol Hepatol. 2019. PMID: 31127189 No abstract available.
-
In vitro intestine model for gut microbiome.Nat Methods. 2019 Jul;16(7):578. doi: 10.1038/s41592-019-0489-5. Nat Methods. 2019. PMID: 31249420 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

