Bioluminescent-based imaging and quantification of glucose uptake in vivo
- PMID: 31086341
- PMCID: PMC6546603
- DOI: 10.1038/s41592-019-0421-z
Bioluminescent-based imaging and quantification of glucose uptake in vivo
Abstract
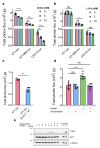
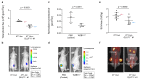
Glucose is a major source of energy for most living organisms, and its aberrant uptake is linked to many pathological conditions. However, our understanding of disease-associated glucose flux is limited owing to the lack of robust tools. To date, positron-emission tomography imaging remains the gold standard for measuring glucose uptake, and no optical tools exist for non-invasive longitudinal imaging of this important metabolite in in vivo settings. Here, we report the development of a bioluminescent glucose-uptake probe for real-time, non-invasive longitudinal imaging of glucose absorption both in vitro and in vivo. In addition, we demonstrate that the sensitivity of our method is comparable with that of commonly used 18F-FDG-positron-emission-tomography tracers and validate the bioluminescent glucose-uptake probe as a tool for the identification of new glucose transport inhibitors. The new imaging reagent enables a wide range of applications in the fields of metabolism and drug development.
Conflict of interest statement
Authors declare no competing interest.
Figures


Comment in
-
Elena Goun.Nat Methods. 2019 Jun;16(6):449. doi: 10.1038/s41592-019-0429-4. Nat Methods. 2019. PMID: 31086340 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

