Light-sheet microscopy in the near-infrared II window
- PMID: 31086342
- PMCID: PMC6579541
- DOI: 10.1038/s41592-019-0398-7
Light-sheet microscopy in the near-infrared II window
Abstract
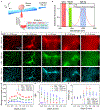
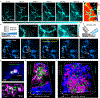
Non-invasive deep-tissue three-dimensional optical imaging of live mammals with high spatiotemporal resolution is challenging owing to light scattering. We developed near-infrared II (1,000-1,700 nm) light-sheet microscopy with excitation and emission of up to approximately 1,320 nm and 1,700 nm, respectively, for optical sectioning at a penetration depth of approximately 750 μm through live tissues without invasive surgery and at a depth of approximately 2 mm in glycerol-cleared brain tissues. Near-infrared II light-sheet microscopy in normal and oblique configurations enabled in vivo imaging of live mice through intact tissue, revealing abnormal blood flow and T-cell motion in tumor microcirculation and mapping out programmed-death ligand 1 and programmed cell death protein 1 in tumors with cellular resolution. Three-dimensional imaging through the intact mouse head resolved vascular channels between the skull and brain cortex, and allowed monitoring of recruitment of macrophages and microglia to the traumatic brain injury site.
Conflict of interest statement
Competing Financial Interests Statement
Authors declare no competing interests.
Figures


References
-
- Helmchen F & Denk W Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005). - PubMed
-
- Lapadula G, Bourdolle A, Allouche F et al. Near-IR two photon microscopy imaging of silica nanoparticles functionalized with isolated sensitized Yb(III) centers. Chem. Mater 26, 1062–1073 (2014).
-
- Alifu N, Yan LL, Zhang HQ et al. Organic dye doped nanoparticles with NIR emission and biocompatibility for ultra-deep in vivo two-photon microscopy under 1040 nm femtosecond excitation. Dyes Pigments 143, 76–85 (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

