Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1
- PMID: 31086348
- PMCID: PMC6642642
- DOI: 10.1038/s41591-019-0440-4
Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1
Abstract
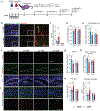
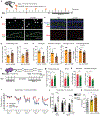
An aged circulatory environment can activate microglia, reduce neural precursor cell activity and impair cognition in mice. We hypothesized that brain endothelial cells (BECs) mediate at least some of these effects. We observe that BECs in the aged mouse hippocampus express an inflammatory transcriptional profile with focal upregulation of vascular cell adhesion molecule 1 (VCAM1), a protein that facilitates vascular-immune cell interactions. Concomitantly, levels of the shed, soluble form of VCAM1 are prominently increased in the plasma of aged humans and mice, and their plasma is sufficient to increase VCAM1 expression in cultured BECs and the hippocampi of young mice. Systemic administration of anti-VCAM1 antibody or genetic ablation of Vcam1 in BECs counteracts the detrimental effects of plasma from aged individuals on young brains and reverses aging aspects, including microglial reactivity and cognitive deficits, in the brains of aged mice. Together, these findings establish brain endothelial VCAM1 at the blood-brain barrier as a possible target to treat age-related neurodegeneration.
Figures

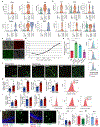
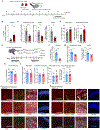
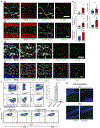
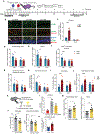
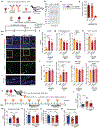


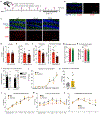

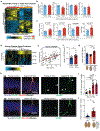
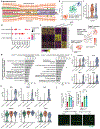


Comment in
-
Targeting VCAM1 rejuvenates the brain in aged mice.Nat Rev Immunol. 2019 Jul;19(7):415. doi: 10.1038/s41577-019-0183-y. Nat Rev Immunol. 2019. PMID: 31127247 No abstract available.
-
Relaying ageing signals.Nat Rev Neurosci. 2019 Jul;20(7):378-379. doi: 10.1038/s41583-019-0190-9. Nat Rev Neurosci. 2019. PMID: 31138911 No abstract available.
References
References (Main Text)
REFERENCES (Methods-only)
-
- Engelhardt B, Vajkoczy P & Weller RO The movers and shapers in immune privilege of the CNS. Nat. Immunol 18, 123–131 (2017). - PubMed
-
- Banks WA From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov 15, 275–292 (2016). - PubMed
-
- Muggeo VMR Estimating regression models with unknown break-points. Stat. Med 22, 3055–71 (2003). - PubMed
-
- Muggeo VMR segmented: An R package to Fit Regression Models with Broken-Line Relationships. R News 8, 20–25 (2008).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

