Simple nutrients bypass the requirement for HLH-30 in coupling lysosomal nutrient sensing to survival
- PMID: 31086360
- PMCID: PMC6516633
- DOI: 10.1371/journal.pbio.3000245
Simple nutrients bypass the requirement for HLH-30 in coupling lysosomal nutrient sensing to survival
Abstract
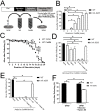
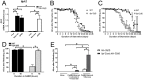
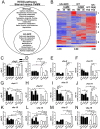
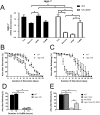
Lysosomes are ubiquitous acidified organelles that degrade intracellular and extracellular material trafficked via multiple pathways. Lysosomes also sense cellular nutrient levels to regulate target of rapamycin (TOR) kinase, a signaling enzyme that drives growth and suppresses activity of the MiT/TFE family of transcription factors that control biogenesis of lysosomes. In this study, we subjected worms lacking basic helix-loop-helix transcription factor 30 (hlh-30), the Caenorhabditis elegans MiT/TFE ortholog, to starvation followed by refeeding to understand how this pathway regulates survival with variable nutrient supply. Loss of HLH-30 markedly impaired survival in starved larval worms and recovery upon refeeding bacteria. Remarkably, provision of simple nutrients in a completely defined medium (C. elegans maintenance medium [CeMM]), specifically glucose and linoleic acid, restored lysosomal acidification, TOR activation, and survival with refeeding despite the absence of HLH-30. Worms deficient in lysosomal lipase 2 (lipl-2), a lysosomal enzyme that is transcriptionally up-regulated in starvation in an HLH-30-dependent manner, also demonstrated increased mortality with starvation-refeeding that was partially rescued with glucose, suggesting a critical role for LIPL-2 in lipid metabolism under starvation. CeMM induced transcription of vacuolar proton pump subunits in hlh-30 mutant worms, and knockdown of vacuolar H+-ATPase 12 (vha-12) and its upstream regulator, nuclear hormone receptor 31 (nhr-31), abolished the rescue with CeMM. Loss of Ras-related GTP binding protein C homolog 1 RAGC-1, the ortholog for mammalian RagC/D GTPases, conferred starvation-refeeding lethality, and RAGC-1 overexpression was sufficient to rescue starved hlh-30 mutant worms, demonstrating a critical need for TOR activation with refeeding. These results show that HLH-30 activation is critical for sustaining survival during starvation-refeeding stress via regulating TOR. Glucose and linoleic acid bypass the requirement for HLH-30 in coupling lysosome nutrient sensing to survival.
Conflict of interest statement
None
Figures




Comment in
-
Surviving starvation simply without TFEB.PLoS Biol. 2019 May 28;17(5):e3000285. doi: 10.1371/journal.pbio.3000285. eCollection 2019 May. PLoS Biol. 2019. PMID: 31136567 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL107594/HL/NHLBI NIH HHS/United States
- R01 NS094692/NS/NINDS NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- T32 HL007081/HL/NHLBI NIH HHS/United States
- R56 AG026561/AG/NIA NIH HHS/United States
- I01 BX000448/BX/BLRD VA/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- R01 AG026561/AG/NIA NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- R01 GM068598/GM/NIGMS NIH HHS/United States
- P40 OD010440/OD/NIH HHS/United States
- K08 HL138262/HL/NHLBI NIH HHS/United States
- I01 BX004235/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

