Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab
- PMID: 31086392
- PMCID: PMC6516638
- DOI: 10.1371/journal.pone.0216954
Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab
Abstract
Background: Blocking the PD-1 pathway induces immune-related adverse events (irAEs) which often involve the thyroid gland (thyroid irAEs). Clinical features of a thyroid irAE including its predictability and relationship to prognosis remain to be elucidated.
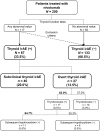
Methods: Two hundred consecutive patients treated with nivolumab at Kyoto University Hospital between September 1, 2014 and August 31, 2017 were included in a retrospective cohort study. We systematically determined and classified subclinical and overt thyroid irAEs based on data collected of serum free T4 and TSH levels. Baseline characteristics and detailed clinical data were analyzed, and analyses of overall survival (OS) excluded patients censored within 1 month from the first administration of nivolumab.
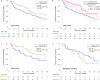
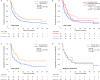
Results: Sixty-seven patients (33.5%) developed thyroid irAEs and these were divided into a subclinical thyroid irAE group (n = 40, 20.0%) and an overt thyroid irAE group (n = 27, 13.5%). Patients with thyroid uptake of FDG-PET before treatment showed high incidences of overt thyroid irAE (adjusted odds ratio 14.48; 95% confidence interval [CI] 3.12-67.19), while the same relationship was not seen with subclinical thyroid irAE. Regarding the total cohort, the thyroid irAE (+) group had a significantly longer median OS than the thyroid irAE (-) group (16.1 versus 13.6 months, hazard ratio [HR] 0.61; 95% CI 0.39-0.93). In 112 non-excluded patients with lung cancer, the thyroid irAE (+) group similarly had a longer median OS than the thyroid irAE (-) group (not reached versus 14.2 months, HR 0.51; 95% CI 0.27-0.92). However, this observation was not seen in 41 non-excluded patients with malignant melanoma (12.0 versus 18.3 months, HR 1.54; 95% CI 0.67-3.43).
Conclusions: By thyroid uptake of FDG-PET, overt thyroid irAEs could be predicted before nivolumab therapy. Thyroid irAEs related to good prognosis in lung cancer but might be inconclusive in malignant melanoma.
Conflict of interest statement
KYH received lecture fees from Ono Pharmaceutical Co., Ltd. and Bristol-Myers Squibb, and research funding from Ono Pharmaceutical Co., Ltd. KYH received lecture fees from Ono Pharmaceutical Co., Ltd. and Bristol-Myers Squibb, and research funding from Ono Pharmaceutical Co., Ltd.. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures