A combination of curcumin, vorinostat and silibinin reverses A β-induced nerve cell toxicity via activation of AKT-MDM2-p53 pathway
- PMID: 31086728
- PMCID: PMC6487801
- DOI: 10.7717/peerj.6716
A combination of curcumin, vorinostat and silibinin reverses A β-induced nerve cell toxicity via activation of AKT-MDM2-p53 pathway
Abstract
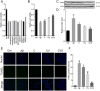
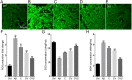
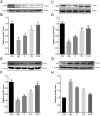
Alzheimer's disease (AD) is a significant health issue for the elderly and becoming increasingly common as the global population ages. Although many efforts have been made to elucidate its pathology, there is still a lack of effective clinical anti-AD agents. Previous research has shown the neuroprotective properties of a combination of curcumin and vorinostat. In this study, nine other neuroprotective agents were investigated to examine whether a three-drug combination of curcumin, vorinostat, and a new drug is more advantageous than the previous two-drug combination in alleviating amyloid beta (Aβ)-induced nerve cell toxicity. Cell viability assay was performed to screen these agents, and further validation tests, including determination of cellular oxidative stress, apoptosis, and activity of the AKT/MDM2/p53 pathway, were performed. Among the nine candidate compounds, only silibinin at 1 µM reduced Aβ25-35-induced toxicity in PC12 cells. The neuroprotective effects of 1 µM silibinin in combination with 5 µM curcumin and 0.5 µM vorinostat (CVS) was shown in PC12 cells, in which it decreased apoptosis and oxidative stress marker levels that were increased by 20 µM Aβ25-35. Western blotting results showed that CVS pretreatment significantly increased the phosphorylation of AKT, BAD, and MDM2, which resulted in decreased intracellular expression of p53. Further, immunofluorescence results showed reduced p53 levels in the nuclei of PC12 cells following CVS pretreatment, indicating a reduction in the p53-mediated transcriptional activity associated with Aβ25-35 exposure. In conclusion, our findings suggested that pretreatment with CVS protected PC12 cells from Aβ25-35-induced toxicity through modulation of the AKT/MDM2/p53 pathway. Thus, CVS may present a new therapeutic option for treating AD.
Keywords: Alzheimer’s disease; Amyloid beta; Curcumin; Silibinin; Vorinostat.
Conflict of interest statement
The authors declare there are no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

