The mitogen-activated protein kinase 4-phosphorylated heat shock factor A4A regulates responses to combined salt and heat stresses
- PMID: 31086987
- PMCID: PMC6760271
- DOI: 10.1093/jxb/erz217
The mitogen-activated protein kinase 4-phosphorylated heat shock factor A4A regulates responses to combined salt and heat stresses
Erratum in
-
Corrigendum to: The mitogen-activated protein kinase 4-phosphorylated heat shock factor A4A regulates responses to combined salt and heat stresses.J Exp Bot. 2021 Jul 28;72(15):5781. doi: 10.1093/jxb/eraa552. J Exp Bot. 2021. PMID: 34180987 Free PMC article. No abstract available.
Abstract
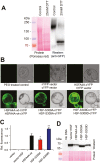
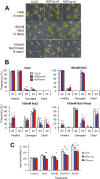
Heat shock factors regulate responses to high temperature, salinity, water deprivation, or heavy metals. Their function in combinations of stresses is, however, not known. Arabidopsis HEAT SHOCK FACTOR A4A (HSFA4A) was previously reported to regulate responses to salt and oxidative stresses. Here we show, that the HSFA4A gene is induced by salt, elevated temperature, and a combination of these conditions. Fast translocation of HSFA4A tagged with yellow fluorescent protein from cytosol to nuclei takes place in salt-treated cells. HSFA4A can be phosphorylated not only by mitogen-activated protein (MAP) kinases MPK3 and MPK6 but also by MPK4, and Ser309 is the dominant MAP kinase phosphorylation site. In vivo data suggest that HSFA4A can be the substrate of other kinases as well. Changing Ser309 to Asp or Ala alters intramolecular multimerization. Chromatin immunoprecipitation assays confirmed binding of HSFA4A to promoters of target genes encoding the small heat shock protein HSP17.6A and transcription factors WRKY30 and ZAT12. HSFA4A overexpression enhanced tolerance to individually and simultaneously applied heat and salt stresses through reduction of oxidative damage. Our results suggest that this heat shock factor is a component of a complex stress regulatory pathway, connecting upstream signals mediated by MAP kinases MPK3/6 and MPK4 with transcription regulation of a set of stress-induced target genes.
Keywords: Arabidopsis; MAP kinases; combined stress; heat; heat shock factor A4A; phosphorylation; promoter binding; salinity; transcription regulation.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures





References
-
- Anckar J, Sistonen L. 2011. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annual Review of Biochemistry 80, 1089–1115. - PubMed
-
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

