Defense of pyrethrum flowers: repelling herbivores and recruiting carnivores by producing aphid alarm pheromone
- PMID: 31087371
- PMCID: PMC6772172
- DOI: 10.1111/nph.15869
Defense of pyrethrum flowers: repelling herbivores and recruiting carnivores by producing aphid alarm pheromone
Abstract
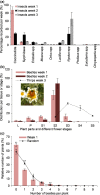
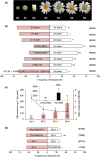
(E)-β-Farnesene (EβF) is the predominant constituent of the alarm pheromone of most aphid pest species. Moreover, natural enemies of aphids use EβF to locate their aphid prey. Some plant species emit EβF, potentially as a defense against aphids, but field demonstrations are lacking. Here, we present field and laboratory studies of flower defense showing that ladybird beetles are predominantly attracted to young stage-2 pyrethrum flowers that emitted the highest and purest levels of EβF. By contrast, aphids were repelled by EβF emitted by S2 pyrethrum flowers. Although peach aphids can adapt to pyrethrum plants in the laboratory, aphids were not recorded in the field. Pyrethrum's (E)-β-farnesene synthase (EbFS) gene is strongly expressed in inner cortex tissue surrounding the vascular system of the aphid-preferred flower receptacle and peduncle, leading to elongated cells filled with EβF. Aphids that probe these tissues during settlement encounter and ingest plant EβF, as evidenced by the release in honeydew. These EβF concentrations in honeydew induce aphid alarm responses, suggesting an extra layer of this defense. Collectively, our data elucidate a defensive mimicry in pyrethrum flowers: the developmentally regulated and tissue-specific EβF accumulation and emission both prevents attack by aphids and recruits aphid predators as bodyguards.
Keywords: (E)-β-farnesene synthase; (E)-β-farnesene; aphid honeydew; cortex-specific expression; false alarm.
© 2019 The Authors. New Phytologist © 2019 New Phytologist Trust.
Figures


References
-
- Abassi SA, Birkett MA, Pettersson J, Pickett JA, Wadhams LJ, Woodcock CM. 2000. Response of the seven‐spot ladybird to an aphid alarm pheromone and an alarm pheromone inhibitor is mediated by paired olfactory cells. Journal of Chemical Ecology 26: 1765–1771.
-
- Avé DA, Gregory P, Tingey WM. 1987. Aphid repellent sesquiterpenes in glandular trichomes of Solanum berthaultii and S. tuberosum . Entomologia Experimentalis et Applicata 44: 131–138.
-
- Beale MH, Birkett MA, Bruce TJ, Chamberlain K, Field LM, Huttly AK, Martin JL, Parker R, Phillips AL, Pickett JA et al 2006. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proceedings of the National Academy of Sciences, USA 103: 10509–105013. - PMC - PubMed
-
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Analytical Biochemistry 72: 248–254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

