Continuous non-invasive optical monitoring of cerebral blood flow and oxidative metabolism after acute brain injury
- PMID: 31088234
- PMCID: PMC6681541
- DOI: 10.1177/0271678X19846657
Continuous non-invasive optical monitoring of cerebral blood flow and oxidative metabolism after acute brain injury
Abstract
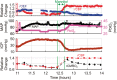
Rapid detection of ischemic conditions at the bedside can improve treatment of acute brain injury. In this observational study of 11 critically ill brain-injured adults, we employed a monitoring approach that interleaves time-resolved near-infrared spectroscopy (TR-NIRS) measurements of cerebral oxygen saturation and oxygen extraction fraction (OEF) with diffuse correlation spectroscopy (DCS) measurement of cerebral blood flow (CBF). Using this approach, we demonstrate the clinical promise of non-invasive, continuous optical monitoring of changes in CBF and cerebral metabolic rate of oxygen (CMRO2). In addition, the optical CBF and CMRO2 measures were compared to invasive brain tissue oxygen tension (PbtO2), thermal diffusion flowmetry CBF, and cerebral microdialysis measures obtained concurrently. The optical CBF and CMRO2 information successfully distinguished between ischemic, hypermetabolic, and hyperemic conditions that arose spontaneously during patient care. Moreover, CBF monitoring during pressor-induced changes of mean arterial blood pressure enabled assessment of cerebral autoregulation. In total, the findings suggest that this hybrid non-invasive neurometabolic optical monitor (NNOM) can facilitate clinical detection of adverse physiological changes in brain injured patients that are otherwise difficult to measure with conventional bedside monitoring techniques.
Keywords: Cerebral blood flow measurement; intrinsic optical imaging; near-infrared spectroscopy; neurocritical care.
Figures




References
-
- Al-Tamimi YZ, Orsi NM, Quinn AC, et al. A review of delayed ischemic neurologic deficit following aneurysmal subarachnoid hemorrhage: historical overview, current treatment, and pathophysiology. World Neurosurg 2010; 73: 654–667. - PubMed
-
- Cunningham A, Salvador R, Coles J, et al. Physiological thresholds for irreversible tissue damage in contusional regions following traumatic brain injury. Brain 2005; 128: 1931–1942. - PubMed
-
- Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies. Stroke 2010; 41: 2391–2395. - PubMed
-
- Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth 2007; 99: 4–9. - PubMed
-
- Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. N Engl J Med 2000; 343: 710–722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

