Shared species of crocodilian trypanosomes carried by tabanid flies in Africa and South America, including the description of a new species from caimans, Trypanosoma kaiowa n. sp
- PMID: 31088523
- PMCID: PMC6515670
- DOI: 10.1186/s13071-019-3463-2
Shared species of crocodilian trypanosomes carried by tabanid flies in Africa and South America, including the description of a new species from caimans, Trypanosoma kaiowa n. sp
Abstract
Background: The genus Trypanosoma Gruby, 1843 is constituted by terrestrial and aquatic phylogenetic lineages both harboring understudied trypanosomes from reptiles including an increasing diversity of crocodilian trypanosomes. Trypanosoma clandestinus Teixeira & Camargo, 2016 of the aquatic lineage is transmitted by leeches to caimans. Trypanosoma grayi Novy, 1906 of the terrestrial lineage is transmitted by tsetse flies to crocodiles in Africa, but the vectors of Neotropical caiman trypanosomes nested in this lineage remain unknown.
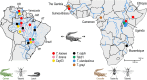
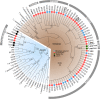
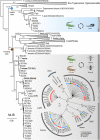
Results: Our phylogenetic analyses uncovered crocodilian trypanosomes in tabanids from South America and Africa, and trypanosomes other than T. grayi in tsetse flies. All trypanosomes found in tabanids clustered in the crocodilian clade (terrestrial lineage) forming six clades: Grayi (African trypanosomes from crocodiles and tsetse flies); Ralphi (trypanosomes from caimans, African and Brazilian tabanids and tsetse flies); Terena (caimans); Cay03 (caimans and Brazilian tabanids); and two new clades, Tab01 (Brazilian tabanid and tsetse flies) and Kaiowa. The clade Kaiowa comprises Trypanosoma kaiowa n. sp. and trypanosomes from African and Brazilian tabanids, caimans, tsetse flies and the African dwarf crocodile. Trypanosoma kaiowa n. sp. heavily colonises tabanid guts and differs remarkably in morphology from other caiman trypanosomes. This species multiplied predominantly as promastigotes on log-phase cultures showing scarce epimastigotes and exhibited very long flagellates in old cultures. Analyses of growth behavior revealed that insect cells allow the intracellular development of Trypanosoma kaiowa n. sp.
Conclusions: Prior to this description of Trypanosoma kaiowa n. sp., no crocodilian trypanosome parasitic in tabanid flies had been cultured, morphologically examined by light, scanning and transmission microscopy, and phylogenetically compared with other crocodilian trypanosomes. Additionally, trypanosomes thought to be restricted to caimans were identified in Brazilian and African tabanids, tsetse flies and the dwarf crocodile. Similar repertoires of trypanosomes found in South American caimans, African crocodiles and tabanids from both continents support the recent diversification of these transcontinental trypanosomes. Our findings are consistent with trypanosome host-switching likely mediated by tabanid flies between caimans and transoceanic migrant crocodiles co-inhabiting South American wetlands at the Miocene.
Keywords: Caiman; Crocodile; Evolution; Morphology; Tabanids; Taxonomy; Transoceanic dispersal; Tsetse flies.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Hoare C. The trypanosomes of mammals: a zoological monograph. Oxford: Blackwell Scientific Publications; 1972.
-
- Telford S., Jr . Parasitic protozoa. In: Kreir JP, editor. The kinetoplastid hemoflagellates of reptiles. New York: Academic Press; 1995. pp. 161–223.
-
- Hoare C. Studies on Trypanosoma grayi II. Experimental transmission to the crocodile. Trans R Soc Trop Med Hyg. 1929;23:39–56. doi: 10.1016/S0035-9203(29)90831-2. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

