Langerhans Cells Sense Staphylococcus aureus Wall Teichoic Acid through Langerin To Induce Inflammatory Responses
- PMID: 31088921
- PMCID: PMC6520447
- DOI: 10.1128/mBio.00330-19
Langerhans Cells Sense Staphylococcus aureus Wall Teichoic Acid through Langerin To Induce Inflammatory Responses
Abstract
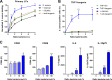
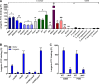
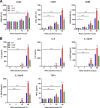
Staphylococcus aureus is a major cause of skin and soft tissue infections and aggravator of the inflammatory skin disease atopic dermatitis (AD [eczema]). Epicutaneous exposure to S. aureus induces Th17 responses through skin Langerhans cells (LCs), which paradoxically contribute to host defense but also to AD pathogenesis. The molecular mechanisms underlying the interaction between S. aureus and LCs are poorly understood. Here we demonstrate that human LCs directly interact with S. aureus through the pattern recognition receptor langerin (CD207). Human, but not mouse, langerin interacts with S. aureus through the conserved β-N-acetylglucosamine (GlcNAc) modifications on wall teichoic acid (WTA), thereby discriminating S. aureus from other staphylococcal species. Importantly, the specific S. aureus WTA glycoprofile strongly influences the level of proinflammatory cytokines that are produced by in vitro-generated LCs. Finally, in a murine epicutaneous infection model, S. aureus strongly upregulated transcripts of Cxcl1, Il6, and Il17, which required the presence of both human langerin and WTA β-GlcNAc. Our findings provide molecular insight into the unique proinflammatory capacities of S. aureus in relation to skin inflammation.IMPORTANCE The bacterium Staphylococcus aureus is an important cause of skin infections and is also associated with the occurrence and severity of eczema. Langerhans cells (LCs), a specific subset of skin immune cells, participate in the immune response to S. aureus, but it is yet unclear how LCs recognize S. aureus Therefore, we investigated the molecular mechanism underlying the interaction between LCs and S. aureus We identified that wall teichoic acid, an abundant polymer on the S. aureus surface, is recognized by langerin, a receptor unique to LCs. This interaction allows LCs to discriminate S. aureus from other related staphylococcal species and initiates a proinflammatory response similar to that observed in patients with eczema. Our data therefore provide important new insights into the relationship between S. aureus, LCs, and eczema.
Keywords: Langerhans cell; Staphylococcus aureus; atopic dermatitis; glycosylation; langerin; wall teichoic acid.
Copyright © 2019 van Dalen et al.
Figures

References
-
- Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV, Latif H, Kim JN, Lockhart A, Artis K, David G, Taylor P, Streib J, Dorrestein PC, Grier A, Gill SR, Zengler K, Hata TR, Leung DY, Gallo RL. 2017. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 9:eaah4680. doi:10.1126/scitranslmed.aah4680. - DOI - PMC - PubMed
-
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, NISC Comparative Sequence Program, Murray PR, Turner ML, Segre JA. 2012. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22:850–859. doi:10.1101/gr.131029.111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
