Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis
- PMID: 31089128
- PMCID: PMC6517419
- DOI: 10.1038/s41467-019-09884-6
Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis
Abstract
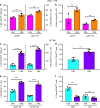
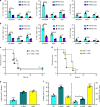
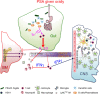
The gut commensal Bacteroides fragilis or its capsular polysaccharide A (PSA) can prevent various peripheral and CNS sterile inflammatory disorders. Fatal herpes simplex encephalitis (HSE) results from immune pathology caused by uncontrolled invasion of the brainstem by inflammatory monocytes and neutrophils. Here we assess the immunomodulatory potential of PSA in HSE by infecting PSA or PBS treated 129S6 mice with HSV1, followed by delayed Acyclovir (ACV) treatment as often occurs in the clinical setting. Only PSA-treated mice survived, with dramatically reduced brainstem inflammation and altered cytokine and chemokine profiles. Importantly, PSA binding by B cells is essential for induction of regulatory CD4+ and CD8+ T cells secreting IL-10 to control innate inflammatory responses, consistent with the lack of PSA mediated protection in Rag-/-, B cell- and IL-10-deficient mice. Our data reveal the translational potential of PSA as an immunomodulatory symbiosis factor to orchestrate robust protective anti-inflammatory responses during viral infections.
Conflict of interest statement
E.M.C., C.R. and S.K.M. are inventors of patent application: PCT/US2016/036803, which describes use of PSA as a treatment for viral inflammatory diseases. The remaining authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

