A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds
- PMID: 31089131
- PMCID: PMC6517429
- DOI: 10.1038/s41467-019-10004-7
A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds
Abstract
Uncontrollable bleeding is a major problem in surgical procedures and after major trauma. Existing hemostatic agents poorly control hemorrhaging from traumatic arterial and cardiac wounds because of their weak adhesion to wet and mobile tissues. Here we design a photo-reactive adhesive that mimics the extracellular matrix (ECM) composition. This biomacromolecule-based matrix hydrogel can undergo rapid gelling and fixation to adhere and seal bleeding arteries and cardiac walls after UV light irradiation. These repairs can withstand up to 290 mm Hg blood pressure, significantly higher than blood pressures in most clinical settings (systolic BP 60-160 mm Hg). Most importantly, the hydrogel can stop high-pressure bleeding from pig carotid arteries with 4~ 5 mm-long incision wounds and from pig hearts with 6 mm diameter cardiac penetration holes. Treated pigs survived after hemostatic treatments with this hydrogel, which is well-tolerated and appears to offer significant clinical advantage as a traumatic wound sealant.
Conflict of interest statement
The authors declare no competing interests.
Figures
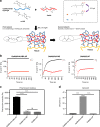

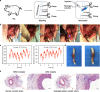

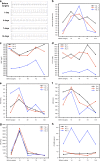
References
-
- Laurenti JB, et al. Enhanced pro-coagulant hemostatic agents based on nanometric zeolites. Micro. Mesopor Mat. 2017;239:263–271. doi: 10.1016/j.micromeso.2016.10.020. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- 2017YFA0104902/National Natural Science Foundation of China (National Science Foundation of China)/International
- 81672162/National Natural Science Foundation of China (National Science Foundation of China)/International
- 81630065/National Natural Science Foundation of China (National Science Foundation of China)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

