Transcriptional Response and Morphological Features of the Neurovascular Unit and Associated Extracellular Matrix After Experimental Stroke in Mice
- PMID: 31089963
- PMCID: PMC6815284
- DOI: 10.1007/s12035-019-1604-4
Transcriptional Response and Morphological Features of the Neurovascular Unit and Associated Extracellular Matrix After Experimental Stroke in Mice
Abstract
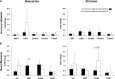
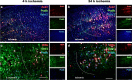
Experimental stroke studies yielded insights into single reactions of the neurovascular unit (NVU) and associated extracellular matrix (ECM). However, the extent of simultaneous processes caused by ischemia and their underlying transcriptional changes are still poorly understood. Strictly following the NVU and ECM concept, this study explored transcriptional responses of cellular and non-cellular components as well as their morphological characteristics following ischemia. Mice were subjected to 4 or 24 h of unilateral middle cerebral artery occlusion. In the neocortex and the striatum, cytoskeletal and glial elements as well as blood-brain barrier and ECM components were analyzed using real-time PCR. Western blot analyses allowed characterization of protein levels and multiple immunofluorescence labeling enabled morphological assessment. Out of 37 genes analyzed, the majority exhibited decreased mRNA levels in ischemic areas, while changes occurred as early as 4 h after ischemia. Down-regulated mRNA levels were predominantly localized in the neocortex, such as the structural elements α-catenin 2, N-cadherin, β-catenin 1, and βIII-tubulin, consistently decreasing 4 and 24 h after ischemia. However, a few genes, e.g., claudin-5 and Pcam1, exhibited increased mRNA levels after ischemia. For several components such as βIII-tubulin, N-cadherin, and β-catenin 1, matching transcriptional and immunofluorescence signals were obtained, whereas a few markers including neurofilaments exhibited opposite directions. In conclusion, the variety in gene regulation emphasizes the complexity of interactions within the ischemia-affected NVU and ECM. These data might help to focus future research on a set of highly sensitive elements, which might prospectively facilitate neuroprotective strategies beyond the traditional single target perspective.
Keywords: Extracellular matrix; Focal cerebral ischemia; Gene expression; Neurovascular unit; Stroke.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

