Apixaban: A Clinical Pharmacokinetic and Pharmacodynamic Review
- PMID: 31089975
- PMCID: PMC6769096
- DOI: 10.1007/s40262-019-00775-z
Apixaban: A Clinical Pharmacokinetic and Pharmacodynamic Review
Abstract
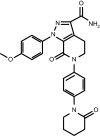
Apixaban is an oral, direct factor Xa inhibitor that inhibits both free and clot-bound factor Xa, and has been approved for clinical use in several thromboembolic disorders, including reduction of stroke risk in non-valvular atrial fibrillation, thromboprophylaxis following hip or knee replacement surgery, the treatment of deep vein thrombosis or pulmonary embolism, and prevention of recurrent deep vein thrombosis and pulmonary embolism. The absolute oral bioavailability of apixaban is ~ 50%. Food does not have a clinically meaningful impact on the bioavailability. Apixaban exposure increases dose proportionally for oral doses up to 10 mg. Apixaban is rapidly absorbed, with maximum concentration occurring 3-4 h after oral administration, and has a half-life of approximately 12 h. Elimination occurs via multiple pathways including metabolism, biliary excretion, and direct intestinal excretion, with approximately 27% of total apixaban clearance occurring via renal excretion. The pharmacokinetics of apixaban are consistent across a broad range of patients, and apixaban has limited clinically relevant interactions with most commonly prescribed medications, allowing for fixed dosages without the need for therapeutic drug monitoring. The pharmacodynamic effect of apixaban is closely correlated with apixaban plasma concentration. This review provides a summary of the pharmacokinetic, pharmacodynamic, biopharmaceutical, and drug-drug interaction profiles of apixaban. Additionally, the population-pharmacokinetic analyses of apixaban in both healthy subjects and in the target patient populations are discussed.
Conflict of interest statement
Wonkyung Byon and Rebecca A. Boyd are current or former employees of Pfizer, Inc., and own stock/stock options. Samira Garonzik is an employee of Bristol-Myers Squibb. Charles E. Frost is an employee of, owns stock/stock options in, has received travel support and payment for lectures from, and is an inventor on patents with Bristol-Myers Squibb.
Figures


References
-
- Bristol-Myers Squibb. Coumadin (wafarin sodium) prescribing information. 2017. http://packageinserts.bms.com/pi/pi_coumadin.pdf. Accessed 2 Feb 2018.
-
- Bristol-Myers Squibb, Pfizer EEIG. EU summary of product characteristics: Eliquis (apixaban tablets). 2018. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info.... Accessed 8 Apr 2019.
-
- Bristol-Myers Squibb Company PI. Eliquis (apixaban) prescribing information. 2018. http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed 14 Jun 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

