Association of Ambulatory Hemodynamic Monitoring of Heart Failure With Clinical Outcomes in a Concurrent Matched Cohort Analysis
- PMID: 31090869
- PMCID: PMC6537799
- DOI: 10.1001/jamacardio.2019.1384
Association of Ambulatory Hemodynamic Monitoring of Heart Failure With Clinical Outcomes in a Concurrent Matched Cohort Analysis
Erratum in
-
Error in Key Points, Abstract, and Methods sections and Title.JAMA Cardiol. 2019 Jun 1;4(6):601. doi: 10.1001/jamacardio.2019.2121. JAMA Cardiol. 2019. PMID: 31215965 Free PMC article. No abstract available.
Abstract
Importance: In a randomized clinical trial, heart failure (HF) hospitalizations were lower in patients managed with guidance from an implantable pulmonary artery pressure sensor compared with usual care. It remains unclear if ambulatory monitoring could also improve long-term clinical outcomes in real-world practice.
Objective: To determine the association between ambulatory hemodynamic monitoring and rates of HF hospitalization at 12 months in clinical practice.
Design, setting, and participants: This matched cohort study of Medicare beneficiaries used claims data collected between June 1, 2014, and March 31, 2016. Medicare patients who received implants of a pulmonary artery pressure sensor were identified from the 100% Medicare claims database. Each patient who received an implant was matched to a control patient by demographic features, history of HF hospitalization, and number of all-cause hospitalizations. Propensity scoring based on comorbidities (arrhythmia, hypertension, diabetes, pulmonary disease, and renal disease) was used for additional matching. Data analysis was completed from July 2017 through January 2019.
Exposures: Implantable pulmonary artery pressure monitoring system.
Main outcomes and measures: The rates of HF hospitalization were compared using the Andersen-Gill method. Days lost owing to events were compared using a nonparametric bootstrap method.
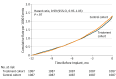
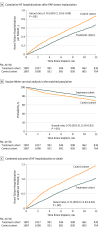
Results: The study cohort consisted of 1087 patients who received an implantable pulmonary artery pressure sensors and 1087 matched control patients. The treatment and control cohorts were well matched by age (mean [SD], 72.7 [10.2] years vs 72.9 [10.1] years) and sex (381 of 1087 female patients [35.1%] in each group), medical history, comorbidities, and timing of preimplant HF hospitalization. At 12 months postimplant, 616 HF hospitalizations occurred in the treatment cohort compared with 784 HF hospitalizations in the control cohort. The rate of HF hospitalization was lower in the treatment cohort at 12 months postimplant (hazard ratio [HR], 0.76 [95% CI, 0.65-0.89]; P < .001). The percentage of days lost to HF hospitalizations or death were lower in the treatment group (HR, 0.73 [95% CI, 0.64-0.84]; P < .001) and the percentage of days lost owing to all-cause hospitalization or death were also lower (HR, 0.77 [95% CI, 0.68-0.88]; P < .001).
Conclusions and relevance: Patients with HF who were implanted with a pulmonary artery pressure sensor had lower rates of HF hospitalization than matched controls and spent more time alive out of hospital. Ambulatory hemodynamic monitoring may improve outcomes in patients with chronic HF.
Conflict of interest statement
Figures
References
-
- Zile MR, Bennett TD, St John Sutton M, et al. . Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118(14):1433-1441. doi:10.1161/CIRCULATIONAHA.108.783910 - DOI - PubMed
-
- Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB; CHAMPION Trial Study Group . Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387(10017):453-461. doi:10.1016/S0140-6736(15)00723-0 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

