Dysregulated Autophagy and Lysosome Function Are Linked to Exosome Production by Micro-RNA 155 in Alcoholic Liver Disease
- PMID: 31090940
- PMCID: PMC7453183
- DOI: 10.1002/hep.30766
Dysregulated Autophagy and Lysosome Function Are Linked to Exosome Production by Micro-RNA 155 in Alcoholic Liver Disease
Abstract
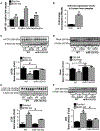
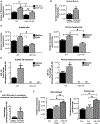
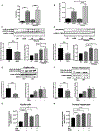
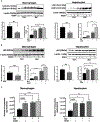
Cellular homeostais, that is normally maintained through autophagy, is disrupted in alcoholic liver disease (ALD). Because autophagy and exosome biogenesis share common elements, we hypothesized that increased exosome production in ALD may be linked to disruption of autophagic function. We found impaired autophagy both in ALD and alcoholic hepatitis (AH) mouse models and human livers with ALD as indicated by increased hepatic p62 and LC3-II levels. Alcohol reduced autophagy flux in vivo in chloroquine-treated mice as well as in vitro in hepatocytes and macrophages treated with bafilomycin A. Our results revealed that alcohol targets multiple steps in the autophagy pathway. Alcohol-related decrease in mechanistic target of rapamycin (mTOR) and Ras homolog enriched in brain (Rheb), that initiate autophagy, correlated with increased Beclin1 and autophagy-related protein 7 (Atg7), proteins involved in phagophore-autophagosome formation, in ALD. We found that alcohol disrupted autophagy function at the lysosomal level through decreased lysosomal-associated membrane protein 1 (LAMP1) and lysosomal-associated membrane protein 2 (LAMP2) in livers with ALD. We identified that micro-RNA 155 (miR-155), that is increased by alcohol, targets mTOR, Rheb, LAMP1, and LAMP2 in the authophagy pathway. Consistent with this, miR-155-deficient mice were protected from alcohol-induced disruption of autophagy and showed attenuated exosome production. Mechanistically, down-regulation of LAMP1 or LAMP2 increased exosome release in hepatocytes and macrophages in the presence and absence of alcohol. These results suggested that the alcohol-induced increase in exosome production was linked to disruption of autophagy and impaired autophagosome and lysosome function. Conclusion: Alcohol affects multiple genes in the autophagy pathway and impairs autophagic flux at the lysosome level in ALD. Inhibition of LAMP1 and LAMP2 promotes exosome release in ALD. We identified miR-155 as a mediator of alcohol-related regulation of autophagy and exosome production in hepatocytes and macrophages.
© 2019 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Potential conflict of interest: Dr. Szabo consults for and received grants from Allergan. She consultsfor Terra Firma, Glympse Bio, Quest, Arrow, GLG, Salix, and Tobira. She received grants from Gilead, Genfit, Intercept, Verlyx, Novartis, SignaBlok, and Shire. She holds intellectual property rights with Up to Date.
Figures





References
-
- Cho HI, Choi JW, Lee SM. Impairment of autophagosome- lysosome fusion contributes to chronic ethanol-induced liver injury. Alcohol 2014;48:717–725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

