Maternal Nanos inhibits Importin-α2/Pendulin-dependent nuclear import to prevent somatic gene expression in the Drosophila germline
- PMID: 31091233
- PMCID: PMC6519790
- DOI: 10.1371/journal.pgen.1008090
Maternal Nanos inhibits Importin-α2/Pendulin-dependent nuclear import to prevent somatic gene expression in the Drosophila germline
Abstract
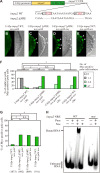
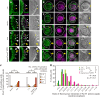
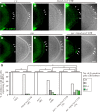
Repression of somatic gene expression in germline progenitors is one of the critical mechanisms involved in establishing the germ/soma dichotomy. In Drosophila, the maternal Nanos (Nos) and Polar granule component (Pgc) proteins are required for repression of somatic gene expression in the primordial germ cells, or pole cells. Pgc suppresses RNA polymerase II-dependent global transcription in pole cells, but it remains unclear how Nos represses somatic gene expression. Here, we show that Nos represses somatic gene expression by inhibiting translation of maternal importin-α2 (impα2) mRNA. Mis-expression of Impα2 caused aberrant nuclear import of a transcriptional activator, Ftz-F1, which in turn activated a somatic gene, fushi tarazu (ftz), in pole cells when Pgc-dependent transcriptional repression was impaired. Because ftz expression was not fully activated in pole cells in the absence of either Nos or Pgc, we propose that Nos-dependent repression of nuclear import of transcriptional activator(s) and Pgc-dependent suppression of global transcription act as a 'double-lock' mechanism to inhibit somatic gene expression in germline progenitors.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Extavour CG, Akam M. Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development (Cambridge, England). 2003;130(24):5869–84. - PubMed
-
- Beams HW, Kessel RG. The problem of germ cell determinants. Int Rev Cytol. 1974;39:413–79. - PubMed
-
- Eddy EM. Germ plasm and the differentiation of the germ cell line. Int Rev Cytol. 1975;43:229–80. - PubMed
-
- Rongo C, Lehmann R. Regulated synthesis, transport and assembly of the Drosophila germ plasm. Trends Genet. 1996;12(3):102–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

