Interplay between proinflammatory cytokines, miRNA, and tissue lesions in Anisakis-infected Sprague-Dawley rats
- PMID: 31091271
- PMCID: PMC6538193
- DOI: 10.1371/journal.pntd.0007397
Interplay between proinflammatory cytokines, miRNA, and tissue lesions in Anisakis-infected Sprague-Dawley rats
Abstract
Background: Anisakiasis is an emerging public health problem, caused by Anisakis spp. nematode larvae. Anisakiasis presents as variable and unspecific gastrointestinal and/or allergic clinical symptoms, which accounts for the high rate of misdiagnosed cases.
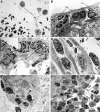
Methodology/principal findings: The aim of this study was to characterize the early cellular (6-72 h p.i.) and molecular (6 h p.i.) immune response and general underlying regulatory mechanism in Anisakis infected rats. Each Sprague-Dawley rat was infected with 10 Anisakis spp. larvae by gastric intubation. Tissues with visible lesions were processed for: i) classic histopathology (HE), immunofluorescence (CD3, iNOS, S100A8/A9), and transmission electron microscopy (TEM); ii) target genes (Il1b, Il6, Il18, Ccl3, Icam1, Mmp9) and microRNA (Rat Immunopathology MIRN-104ZF plate, Quiagen) expression analysis; and iii) global DNA methylation. Histopathology revealed that Anisakis larval migration caused moderate to extensive hemorrhages in submucosal and epimysial/perimysial connective tissue. In stomach and muscle, moderate to abundant mixed inflammatory infiltrate was present, dominated by neutrophils and macrophages, while only mild infiltration was seen in intestine. Lesions were characterized by the presence of CD3+, iNOS+, and S100A8/A9+ cells. The greatest number of iNOS+ and S100A8/A9+ cells was seen in muscle. Il6, Il1b, and Ccl3 showed particularly strong expression in stomach and visceral adipose tissues, but the order of expression differed between tissues. In total, three miRNAs were differentially expressed, two in stomach (miRNA-451 and miRNA-223) and two in intestine (miRNA-451 and miRNA-672). No changes in global DNA methylation were observed in infected tissues relative to controls.
Conclusions/significance: Anisakis infection induces strong immune responses in infected rats with marked induction of specific proinflammatory cytokines and miRNA expression. Deciphering the functional role of these cytokines and miRNAs will help in understanding the anisakiasis pathology and controversies surrounding Anisakis infection in humans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- WHO. First formal meeting of the Foodborne Disease Burden Epidemiology Reference Group (FERG). 2007;
-
- EFSA Panel on Biological Hazards. Scientific opinion on risk assessment of parasites in fishery products. EFSA J. 2010;8: 1543.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

