Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle
- PMID: 31091443
- PMCID: PMC7034941
- DOI: 10.1016/j.celrep.2019.04.074
Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle
Abstract
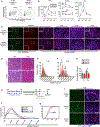
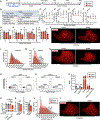
The necessity of mesenchymal stromal cells, called fibroadipogenic progenitors (FAPs), in skeletal muscle regeneration and maintenance remains unestablished. We report the generation of a PDGFRαCreER knockin mouse model that provides a specific means of labeling and targeting FAPs. Depletion of FAPs using Cre-dependent diphtheria toxin expression results in loss of expansion of muscle stem cells (MuSCs) and CD45+ hematopoietic cells after injury and impaired skeletal muscle regeneration. Furthermore, FAP-depleted mice under homeostatic conditions exhibit muscle atrophy and loss of MuSCs, revealing that FAPs are required for the maintenance of both skeletal muscle and the MuSC pool. We also report that local tamoxifen metabolite delivery to target CreER activity in a single muscle, removing potentially confounding systemic effects of ablating PDGFRα+ cells distantly, also causes muscle atrophy. These data establish a critical role of FAPs in skeletal muscle regeneration and maintenance.
Keywords: FAP; Mesenchymal; PDGFRα; fibroadipogenic progenitor; local recombination; muscle; niche; satellite cell; stem cell; stromal.
Published by Elsevier Inc.
Figures
References
-
- Carlson BM, and Faulkner JA (1989). Muscle transplantation between young and old rats: age of host determines recovery. Am. J. Physiol 256, C1262–C1266. - PubMed
-
- Farley FW, Soriano P, Steffen LS, and Dymecki SM (2000). Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28, 106–110. - PubMed
-
- Frontera WR, and Ochala J. (2015). Skeletal muscle: a brief review of structure and function. Calcif. Tissue Int 96, 183–195. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

