Anti-Adipogenic Effects of Delphinidin-3- O- β-Glucoside in 3T3-L1 Preadipocytes and Primary White Adipocytes
- PMID: 31091729
- PMCID: PMC6571603
- DOI: 10.3390/molecules24101848
Anti-Adipogenic Effects of Delphinidin-3- O- β-Glucoside in 3T3-L1 Preadipocytes and Primary White Adipocytes
Abstract
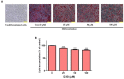
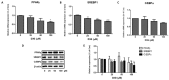
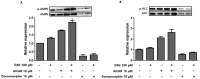
Delphinidin-3-O-β-glucoside (D3G) is a health-promoting anthocyanin whose anti-obesity activity has not yet been thoroughly investigated. We examined the effects of D3G on adipogenesis and lipogenesis in 3T3-L1 adipocytes and primary white adipocytes using real-time RT-PCR and immunoblot analysis. D3G significantly inhibited the accumulation of lipids in a dose-dependent manner without displaying cytotoxicity. In the 3T3-L1 adipocytes, D3G downregulated the expression of key adipogenic and lipogenic markers, which are known as peroxisome proliferator-activated receptor gamma (PPARγ), sterol regulatory element-binding transcription factor 1 (SREBP1), CCAAT/enhancer-binding protein alpha (C/EBPα), and fatty acid synthase (FAS). Moreover, the relative protein expression of silent mating type information regulation 2 homolog 1 (SIRT1) and carnitine palmitoyltransferase-1 (CPT-1) were increased, alongside reduced lipid levels and the presence of several small lipid droplets. Furthermore, D3G increased the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC), which suggests that D3G may play a role in AMPK and ACC activation in adipocytes. Our data indicate that D3G attenuates adipogenesis and promotes lipid metabolism by activating AMPK-mediated signaling, and, hence, could have a therapeutic role in the management and treatment of obesity.
Keywords: 3T3-L1 adipocytes; adipogenesis; anthocyanin; delphinidin-3-O-β-glucoside; differentiation; primary white adipocytes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
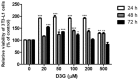



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

