Rheology of Concentrated Polymer/Ionic Liquid Solutions: An Anomalous Plasticizing Effect and a Universality in Nonlinear Shear Rheology
- PMID: 31091730
- PMCID: PMC6572180
- DOI: 10.3390/polym11050877
Rheology of Concentrated Polymer/Ionic Liquid Solutions: An Anomalous Plasticizing Effect and a Universality in Nonlinear Shear Rheology
Abstract
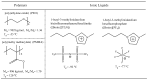
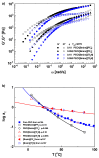
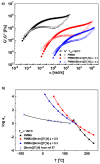
An anomalous plasticizing effect was observed in polymer/ionic liquid (IL) solutions by applying broad range of rheological techniques. Poly(ethylene oxide)(PEO)/IL solutions exhibit stronger dynamic temperature dependence than pure PEO, which is in conflict with the knowledge that lower-Tg solvent increases the fractional free volume. For poly(methy methacrylate)(PMMA)/IL solutions, the subtle anomaly was detected from the fact that the effective glass transition temperature Tg,eff of PMMA in IL is higher than the prediction of the self-concentration model, while in conventional polymer solutions, Tg,eff follows the original Fox equation. Observations in both solutions reveal retarded segmental dynamics, consistent with a recent simulation result (Macromolecules, 2018, 51, 5336) that polymer chains wrap the IL cations by hydrogen bonding interactions and the segmental unwrapping delays their relaxation. Start-up shear and nonlinear stress relaxation tests of polymer/IL solutions follow a universal nonlinear rheological behavior as polymer melts and solutions, indicating that the segment-cation interaction is not strong enough to influence the nonlinear chain orientation and stretch. The present work may arouse the further theoretical, experimental, and simulation interests in interpreting the effect of complex polymer-IL interaction on the dynamics of polymer/IL solutions.
Keywords: linear and nonlinear rheology; plasticizing effect; polymer/ionic liquid solution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Gu Y.Y., Cussler E.L., Lodge T.P. ABA-triblock copolymer ion gels for CO2 separation applications. J. Membr. Sci. 2012;423:20–26. doi: 10.1016/j.memsci.2012.07.011. - DOI
-
- Ranjbaran F., Kamio E., Matsuyama H. Inorganic/organic composite ion gel membrane with high mechanical strength and high CO2 separation performance. J. Membr. Sci. 2017;544:252–260. doi: 10.1016/j.memsci.2017.09.036. - DOI
-
- Ranjbaran F., Kamio E., Matsuyama H. Ion gel membrane with tunable inorganic/organic composite network for CO2 separation. Ind. Eng. Chem. Res. 2017;56:12763–12772. doi: 10.1021/acs.iecr.7b03279. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

