Synthesis and Evaluation of Antidepressant Activities of 5-Aryl-4,5-dihydrotetrazolo [1,5- a]thieno[2,3- e]pyridine Derivatives
- PMID: 31091808
- PMCID: PMC6572050
- DOI: 10.3390/molecules24101857
Synthesis and Evaluation of Antidepressant Activities of 5-Aryl-4,5-dihydrotetrazolo [1,5- a]thieno[2,3- e]pyridine Derivatives
Abstract
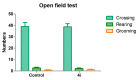
In this study, we synthetized a series of 5-aryl-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine derivatives containing tetrazole and other heterocycle substituents, i.e., triazole, methyltriazole, and triazolone. The forced swim test (FST) and tail suspension test (TST) were used to evaluate the antidepressant activity of the target compounds. The compound 5-[4-(trifluoromethyl)phenyl]-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4i) showed the highest antidepressant activity, with a reduced immobility time of 55.33% when compared with the control group. Using an open-field test, compound 4i was shown to not affect spontaneous activity of mice. The determination of in vivo 5-hydroxytryptamine (5-HT) concentration showed that compound 4i may have an effect in the mouse brain. The biological activities of all synthetized compounds were verified by molecular docking studies. Compound 4i showed significant interactions with residues of the 5-HT1A receptor homology model.
Keywords: 5-HT; FST; antidepressant; molecular docking studies; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Coumarin and 3,4-dihydroquinolinone derivatives: Synthesis, antidepressant activity, and molecular docking studies.Pharmacol Rep. 2019 Dec;71(6):1244-1252. doi: 10.1016/j.pharep.2019.07.011. Epub 2019 Jul 31. Pharmacol Rep. 2019. PMID: 31670061
-
Design, Synthesis, and In Vivo and In Silico Evaluation of Coumarin Derivatives with Potential Antidepressant Effects.Molecules. 2021 Sep 13;26(18):5556. doi: 10.3390/molecules26185556. Molecules. 2021. PMID: 34577028 Free PMC article.
-
Synthesis and biological evaluation of a series of benzoxazole/benzothiazole-containing 2,3-dihydrobenzo[b][1,4]dioxine derivatives as potential antidepressants.Bioorg Med Chem Lett. 2014 Apr 1;24(7):1766-70. doi: 10.1016/j.bmcl.2014.02.031. Epub 2014 Feb 20. Bioorg Med Chem Lett. 2014. PMID: 24618300
-
Synthesis and antidepressant activities of 4-(substituted-phenyl)tetrazolo[1,5-a]quinazolin-5(4H)-ones and their derivatives.Mol Divers. 2015 Nov;19(4):817-28. doi: 10.1007/s11030-015-9623-1. Epub 2015 Aug 7. Mol Divers. 2015. PMID: 26251313
-
The need for guidance in antidepressant drug development: Revisiting the role of the forced swim test and tail suspension test.Regul Toxicol Pharmacol. 2024 Aug;151:105666. doi: 10.1016/j.yrtph.2024.105666. Epub 2024 Jun 27. Regul Toxicol Pharmacol. 2024. PMID: 38942190 Review.
Cited by
-
Study of different heterocycles showing significant anti-severe acute respiratory syndrome 2 activity in vitro and in vivo.Vet World. 2024 Jun;17(6):1281-1290. doi: 10.14202/vetworld.2024.1281-1290. Epub 2024 Jun 14. Vet World. 2024. PMID: 39077461 Free PMC article.
-
Discovery of 4,5,6,7-Tetrahydrothieno [3,2-b] Pyridine as Novel Fungicide Lead Scaffold.Microorganisms. 2025 Jul 5;13(7):1588. doi: 10.3390/microorganisms13071588. Microorganisms. 2025. PMID: 40732097 Free PMC article.
-
Design and synthesis of some novel hybrid molecules based on 4-thiazolidinone bearing pyridine-pyrazole scaffolds: molecular docking and molecular dynamics simulations of its major constituent onto DNA gyrase inhibition.Mol Divers. 2024 Apr;28(2):693-709. doi: 10.1007/s11030-023-10612-y. Epub 2023 Feb 8. Mol Divers. 2024. PMID: 36750538
-
Antidepressant, anxiolytic, antipyretic, and thrombolytic profiling of methanol extract of the aerial part of Piper nigrum: In vivo, in vitro, and in silico approaches.Food Sci Nutr. 2021 Jan 5;9(2):833-846. doi: 10.1002/fsn3.2047. eCollection 2021 Feb. Food Sci Nutr. 2021. PMID: 33598167 Free PMC article.
-
Development of heterocyclic-based frameworks as potential scaffold of 5-HT1A receptor agonist and future perspectives: A review.Medicine (Baltimore). 2024 Jun 14;103(24):e38496. doi: 10.1097/MD.0000000000038496. Medicine (Baltimore). 2024. PMID: 38875413 Free PMC article. Review.
References
-
- Badr S.M.I. Synthesis and anti-inflammatory activity of novel 2,5-disubstituted thiophene derivatives. Turk. J. Chem. 2010;35:131–143.
-
- Amer A.E., Sherif M.H., Assy M.G., Al-Omar M.A., Ragab I. Antiarrhythmic, serotonin antagonist and antianxiety activities of novel substituted thiophene derivatives synthesized from 2-amino-4,5,6,7-tetrahydro-N-phenylbenzo[b]thiophene-3-carboxamide. Eur. J. Med. Chem. 2010;45:5935–5942. doi: 10.1016/j.ejmech.2010.09.059. - DOI - PubMed
-
- Fadda A.A., Abdel-Latif E., El-Mekawy R.E. Synthesis of some new arylazothiophene and arylazopyrazole derivatives as antitumor agents. J. Pharm. Pharmacol. 2012;3:148–157. doi: 10.4236/pp.2012.32022. - DOI
-
- Ali K.A., Abdalghfar H.S., Mahmoud K., Ragab E.A. Synthesis and antitumor activity of new Polysubstituted Thiophenes and 1,3,4-thiadiazoles incorporating 2,6-pyridine moieties. J. Heterocycl. Chem. 2013;50:1157–1164. doi: 10.1002/jhet.1613. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

