Point-of-Care HIV Viral Load Testing: an Essential Tool for a Sustainable Global HIV/AIDS Response
- PMID: 31092508
- PMCID: PMC6589862
- DOI: 10.1128/CMR.00097-18
Point-of-Care HIV Viral Load Testing: an Essential Tool for a Sustainable Global HIV/AIDS Response
Abstract
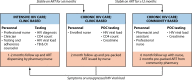
The global public health community has set ambitious treatment targets to end the HIV/AIDS pandemic. With the notable absence of a cure, the goal of HIV treatment is to achieve sustained suppression of an HIV viral load, which allows for immunological recovery and reduces the risk of onward HIV transmission. Monitoring HIV viral load in people living with HIV is therefore central to maintaining effective individual antiretroviral therapy as well as monitoring progress toward achieving population targets for viral suppression. The capacity for laboratory-based HIV viral load testing has increased rapidly in low- and middle-income countries, but implementation of universal viral load monitoring is still hindered by several barriers and delays. New devices for point-of-care HIV viral load testing may be used near patients to improve HIV management by reducing the turnaround time for clinical test results. The implementation of near-patient testing using these new and emerging technologies may be an essential tool for ensuring a sustainable response that will ultimately enable an end to the HIV/AIDS pandemic. In this report, we review the current and emerging technology, the evidence for decentralized viral load monitoring by non-laboratory health care workers, and the additional considerations for expanding point-of-care HIV viral load testing.
Keywords: AIDS; HIV; differentiated care; point-of-care; viral load.
Copyright © 2019 American Society for Microbiology.
Figures



References
-
- UNAIDS. 2018. UNAIDS data 2018. UNAIDS, Geneva, Switzerland.
-
- World Health Organization. 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2nd ed World Health Organization, Geneva, Switzerland. - PubMed
-
- Centers for Disease Control and Prevention. 2018. U=U campaign, 2017. https://www.preventionaccess.org. Accessed 6 September 2018.
-
- UNAIDS. 2014. 90-90-90. An ambitious treatment target to help end the AIDS epidemic. World Health Organization, Geneva, Switzerland: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

